
Life, in its incredible diversity and complexity, is the result of a fascinating interplay of countless biological processes and structures that often operate in the hidden. Every cell in our body, no matter how insignificant it may seem, is a small wonder of nature. It houses a multitude of proteins that, like tiny machines, tirelessly work to preserve life. These proteins not only ensure that our cells perform their daily tasks but also play a crucial role in protecting our bodies.
Our immune system, one of the most impressive creations of evolution, defends us against countless threats – from invading microorganisms to degenerated cells that can endanger our health. This defense system is a complex network of cells, proteins, and chemical signals that together make the difference between health and disease. In this essay, we will focus on this „wonderful world of life”, with a particular emphasis on the immune system. The goal of this work is to provide a comprehensive and accessible understanding of how the immune system functions. It is recommended to read the chapters in the given order so that the information builds upon itself step by step, allowing for a deeper understanding of the topic.
Let us now immerse ourselves in this marvellous world of life to understand how the invisible preserves our lives.
1. A Look Beneath the Skin – A Journey Within
2. The Cell – The Fundamental Building Block
3. Proteins – The Building Blocks of Life
4. From Code to Protein – Cellular Mechanisms
5. The Body’s Shield – Our Immune System
6. Hidden Defense – The Power of Cross-Immunity
7. Key takeaways
8. Closing words
Complete Table of Contents
1. A Look Beneath the Skin – A Journey Within
2. The Cell – The Fundamental Building Block
3. Proteins – The Building Blocks of Life
4. From Code to Protein – Cellular Mechanisms
4.1. From Signals to Actions
4.2. The Protein Biosynthesis
4.2.1. Transcription
4.2.2. Translation
4.3. The Protein
5. The Body’s Shield – Our Immune System
5.1. Origin of Immune Cells
5.2. Mechanisms of Immune Recognition: NON-SELF vs. SELF
5.2. a) SELF-Markers: MHC Molecules
5.2. b) SELF-Markers: CD47 Molecule
5.2. c) SELF-Markers: Sialic Acid
5.3. The Nonspecific Immune Defense
I – First Line of Defense: Mechanical and Chemical Barriers
II – Second Line of Defense: White Blood Cells
5.3. a) Granulocytes
5.3. b) Macrophages
5.3. c) Dendritic Cells
5.3. d) Natural Killer Cells
5.4. The Complement System
5.5. The Specific Immune Defense
5.5.1. Key Players of the Adaptive Immune Response
5.5.1. a) Development and Maturation of Lymphocytes
5.5.1. b) Humoral and Cellular Defense Mechanisms
5.5.1. c) Migration and Distribution of Lymphocytes
5.5.1. d) Structure of the Lymphatic Organs
5.5.2. Naive B and T Cells: The Diversity of the Immune Response
5.5.3. The Role of Antigen-Presenting Cells (APCs)
5.5.3. a) Antigen Presentation via MHC-II Molecules
5.5.3. b) Antigen Presentation via MHC-I Molecules
5.5.3. c) Dendritic Cells Migrate to the Lymph Nodes
5.5.4. The Importance of Lymph Nodes for the Adaptive Immune Response
5.5.5. Recognition Phase
5.5.6. Activation Phase
5.5.6. a) T Cell Activation
5.5.6. b) B Cell Activation
5.5.7. Effector Phase
5.5.7. a) T Helper Cells
5.5.7. b) Cytotoxic T Cells
5.5.7. c) Plasma Cells
III – Third Line of Defense: The Antibodies
5.5.8. Types of Antibodies
5.5.8. a) IgM – The First Antibody
5.5.8. b) Class Switching (Isotype Switching) to IgG
5.5.8. c) IgA – The Protective Barrier of Mucous Membranes
5.5.8. d) Mucosal Immunity: Why IgG Is Unsuitable for This
5.5.9. The Action Phase of Antibodies
5.5.10. Regulatory T Cells and Their Role in the Immune System
5.5.11. Switch-Off Phase
5.5.12. Immunological Memory
5.6. Summary
6. Hidden Defense – The Power of Cross-Immunity
7. Key takeaways
8. Closing words
1. A Look Beneath the Skin – A Journey Within
The organism, the living body of a human being, is a fascinating construct that functions on multiple levels of organization. This organizational structure is the invisible bond that connects the cells, weaves the tissues, and shapes the organs.

To illustrate the complex interplay, let’s zoom in, starting with a look at the organ system level.
Organism ➔ Organ systems
Organ systems are essential units in the human body that combine structure and function. The most important organ systems include the cardiovascular system, the respiratory system, the digestive system, the nervous system, the endocrine system (hormone system), the immune system, the urinary system and the reproductive system. Each of these systems fulfils a unique function that contributes to the overall function of the organism.

The digestive system is responsible for the digestion and absorption of nutrients. The task of the respiratory system is to take in oxygen and remove carbon dioxide. The cardiovascular system moves blood around the body to transport nutrients and oxygen to the cells and remove waste products.
An organ system consists of a single organ or a group of organs that are closely connected. When we zoom in closer, we can examine the individual organs.
Organism ➔ Organ systems ➔ Organs
Organs are important parts of the body that perform specific tasks. Each organ has its own distinct shape and function. The various organs work together like a well-coordinated team. Examples of organs include the heart, lungs, liver, and brain.

The heart pumps blood through the body, while the lungs are responsible for gas exchange. Among many other tasks, the brain controls breathing and the heartbeat. The stomach grinds and mixes food, the intestine extracts nutrients from food and the liver aids digestion and detoxifies.
Organs are made up of different tissues that are closely connected and work together to support the function of the organ. When we zoom in even further, we see the tissues that make up the organ.
Organism ➔ Organ systems ➔ Organs ➔ Tissues
Different tissues in the body fulfil different tasks and are tailored to the needs of the organs they support. By working together, they enable a variety of physiological processes, including movement, sensory perception, digestion and immune defence.
There are four main types of tissues in the human body: muscle tissue, connective tissue, epithelial tissue, and nervous tissue.

To stay with the example of the heart, muscle tissue enables the pumping of blood throughout the body, while nervous tissue controls the electrical signals that regulate heart action. Connective tissue provides structural support and connects the different parts of the heart, while epithelial tissue lines and protects the inner surfaces of the heart.
In the lungs, epithelial tissue facilitates gas exchange. Connective tissue supports the structure and connects the various tissue components. The smooth muscle tissue in the walls of the airways allows for the regulation of airflow. Finally, nervous tissue transmits signals to the brain to control the breathing movements.
Tissues are made up of a variety of cells that come together to perform a specific task. When we zoom in even closer, we can recognize the individual cells.
Organism ➔ Organ systems ➔ Organs ➔ Tissues ➔ Cells
Cells are the fundamental building blocks of tissues. They are often specialized for specific functions within the tissue. They can have different shapes, sizes, and structures, adapted to their respective functions. For example, cells in muscle tissue are specialized for contraction, while cells in epithelial tissue are specialized for protection and absorption.

In addition to the specialized cells that perform specific tasks in the tissue, there is also a special type of cell: stem cells. Unlike other cells in the body, stem cells have the remarkable ability to transform into many different cell types. They are like the „blanks” of the body – not yet committed to a specific task and full of possibilities. When the body needs them, stem cells can become muscle cells, nerve cells, or immune system cells.
Stem Cells
Basically, there are two types of stem cells: embryonic and adult stem cells. Embryonic stem cells are found in the early stages of an embryo, when it is just beginning to develop. These cells have the unique ability to differentiate into virtually any cell type in the human body. They are like the building blocks from which all parts of the body can be constructed.
In the adult body, on the other hand, there are adult stem cells. They are present in various tissues and organs. In contrast to embryonic stem cells, adult stem cells cannot become every cell type in the body. They can only transform into the cell types found in the tissue from which they originate. For example, there are skin stem cells in the skin or liver stem cells in the liver.
What makes these stem cells so special is their ability to repair. If cells in the body are damaged or dead, these stem cells can migrate to this location and ‘build’ new, healthy cells to replace the damaged ones.
Stem cells can divide continuously. This ensures that there is always a supply of stem cells that can differentiate (transform) into the cells the body needs. For example, if liver cells are damaged, liver stem cells can step in, divide and form new, healthy liver cells.
Adult stem cells play an important role in keeping our bodies healthy by producing new cells when needed and repairing damaged tissue.
Cells in tissues communicate and interact with each other via complex signalling pathways and molecules. This communication is crucial for the coordination of cell activities and the maintenance of the function of the tissue as a whole.
A multitude of processes take place in every cell that are crucial for the growth, development, and survival of the organism. Cells are the fundamental building blocks of all living organisms. All life, from the smallest microorganisms to the largest animals, is made up of these tiny yet incredibly complex units. Each cell is an astonishing world of its own, filled with molecules and chemical reactions that make life possible.
One could say: Life begins with the cell.
2. The Cell – The Fundamental Building Block
Life on our planet is a fascinating miracle that begins on a microscopic level in the cell. These tiny building blocks of life are the basis for all living things.
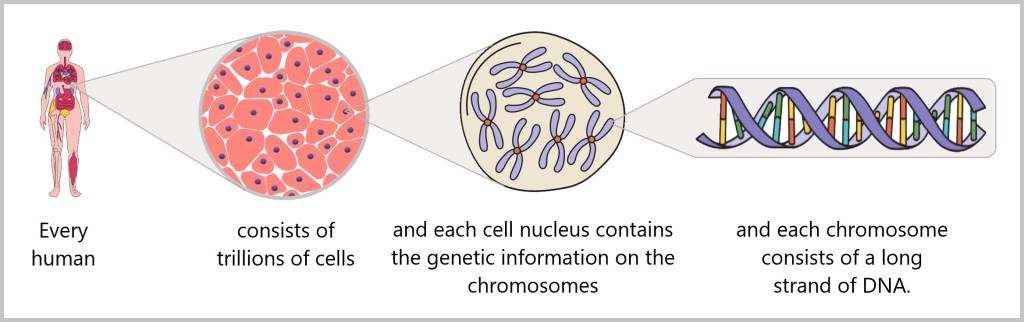
A human being develops from two tiny cells – the egg cell and the sperm cell. In the first few weeks after fertilisation, the embryo undergoes a process in which it turns from a fertilised egg cell into a multitude of cells from which all organs and tissues are formed. By the time the embryo is called a foetus – usually from the eighth week of pregnancy – most of the organs and tissues have been created. From then on, development is mainly focussed on the growth and maturation of these structures. It is estimated that a newborn baby consists of about 20 to 30 trillion cells, while an average adult human has about 30 to 40 trillion cells. A study published in the journal PNAS categorises the human body into 60 tissue systems, 400 major cell types and 1264 separate cell groups.
A brief and concise introduction to cell structure comes from the American digital communication agency Nucleus Medical Media. [Spektrum] It is intended to prepare us for the following explanations.
Although the human body is made up of many different cell types, they all share a similar basic structure. Each cell is surrounded by a protective covering, the cell membrane. Inside the cell, in a gel-like substance called cytoplasm, there are many small, specialized structures known as organelles. One can think of these organelles as tiny machines or workshops within the cell, each performing a specific task.
One of these important organelles is the nucleus. The nucleus acts like the control center of the cell – it contains the genetic information (DNA) and provides instructions to the other organelles on how to function. There is one exception: red blood cells. These cells are so specialized for their task that they no longer have a nucleus.

Each cell organelle performs specific tasks.
Nucleus: The nucleus stores the genetic information of the cell (DNA) and controls all the activities of the cell. It is surrounded by a protective layer (cell membrane).
Nucleolus: The nucleolus is a small structure within the nucleus. This is where ribosomes (molecular workshops) are produced, which are necessary for protein production.
Ribosomes: These small structures produce proteins that are essential for many processes in the cell. They can either float freely in the cytoplasm or be attached to the endoplasmic reticulum.
Endoplasmic Reticulum (ER): The ER is a network of tubules within the cell that transports proteins and lipids (fats) and also partially produces them.
Golgi Apparatus: The Golgi apparatus acts like a dispatch center. It packages and sends proteins and lipids (which come from the ER) to the correct locations within the cell or outside of it.
Mitochondria: These organelles are often referred to as the ‘power stations’ of the cell because they produce the energy that the cell needs to function.
Lysosomes: Lysosomes act like the cell’s garbage disposal. They contain enzymes that break down old cell components and waste materials.
Cytoskeleton: The cytoskeleton is a network of protein fibers that gives the cell its shape, stabilizes it, and also facilitates movement.
Life, as we understand it, fundamentally requires three basic elements. First, a cell membrane that separates the interior of the cell from the external environment. Second, a metabolism is necessary to extract energy from the environment. And third, genetic information is needed to control and regulate vital processes. All living organisms are based on these three principles: cell membrane, metabolism, and information, which must come together to enable life. In this context, the information contained in DNA is the most essential ingredient for life. [Where does life come from? – a documentary in German language]
Hidden in the depths of the cell lies DNA – the secret of life. It reveals itself as an impressive weave of molecules. These molecules carry the genetic code that determines the diversity and complexity of life. This is why DNA is often referred to as the book of life, as it contains the instructions for the development and functioning of every organism on our planet. By reading the DNA, proteins are produced, which are the actual executors of these instructions. Proteins shape structures, catalyze reactions, and transmit signals that enable life in all its forms. They are the building blocks of all living things and the tools that bring us to life.
From cells to DNA to proteins – life is a breathtaking interplay of molecules and processes that give rise to an infinite variety of forms and functions. In every tiny particle that makes up life lies a deep fascination and an immeasurable wonder.
DNA explanation
DNA, or deoxyribonucleic acid, is a biomolecular structure that functions as genetic material in the cells of all living organisms and is located in the nucleus of each cell. It stores all genetic information, serving as the complete blueprint and instruction manual for the structure and function of our bodies.
The DNA is structurally similar to a rope ladder. It consists of two long sides that represent the „strands” of DNA. Each „rung” of the ladder is made up of two nucleotides, the chemical building blocks of DNA, which consist of a base, a sugar (deoxyribose), and a phosphate group. The base pairs function as the rungs, while the strands of DNA hold the ladder-like structure together, alternating between sugar and phosphate.
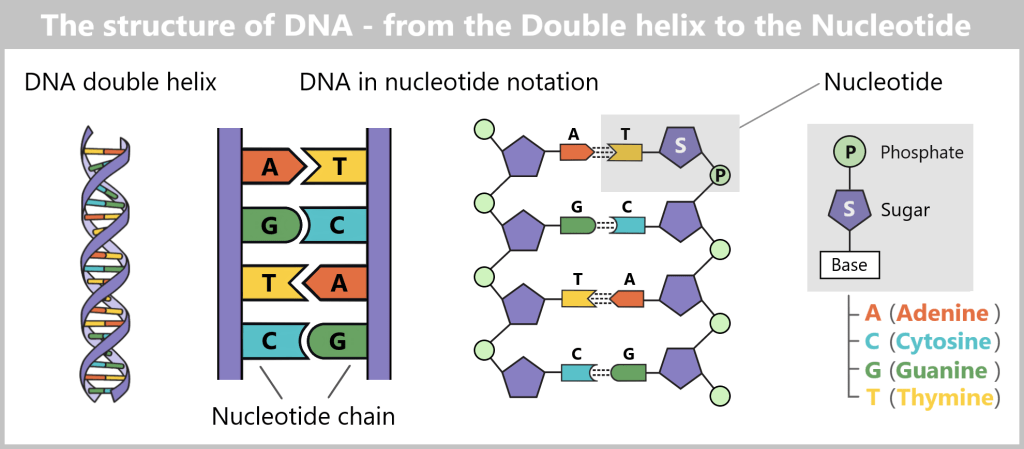
The two nucleotide chains are twisted around their own axis in a helical shape. This structure can be imagined like a twisted ladder or spiral staircase. In biology, this spiral is called the DNA double helix (double because there are two nucleotide chains – double is indeed better). This three-dimensional structure not only gives DNA stability but also saves space. Because if you were to completely unroll the DNA of a human cell, it would measure a remarkable two meters long – life literally hangs by a pretty long but incredibly thin thread!
In DNA, there are four different types of bases: adenine (A), cytosine (C), guanine (G), and thymine (T). These bases always pair in the same way, with adenine (A) binding to thymine (T) and cytosine (C) binding to guanine (G). This binding is complementary, with each DNA strand representing the negative version of the other strand.
The arrangement of these base pairs forms the genetic code, which carries the information for the production of proteins that are necessary for the structure, function and regulation of the body cells, tissues and organs of an organism.
Each gene is a specific segment of DNA that contains the instructions for the formation of a particular protein. Thus, DNA acts as the „cookbook” of life, determining the development and function of an organism and being responsible for the inheritance of traits from one generation to the next. With each cell division, the DNA is copied and passed on to the new cells, ensuring that genetic information is transmitted from generation to generation.
Mutations in the DNA sequence can lead to changes in the structure and function of proteins, which in turn can affect the appearance, health, and behavior of an organism. Some mutations can cause diseases, while others may have no noticeable effects or even be beneficial.
3. Proteins – The Building Blocks of Life
Proteins, which are created by reading the DNA, are the true architects of life. They perform a wide variety of essential functions in the body and are responsible for almost all biological processes. On one hand, proteins serve as structural building blocks and form the foundation for cells, tissues, and organs. They give cells their shape and strength, enabling them to carry out their functions properly.
In addition, proteins are also involved in enzymatic reactions that drive metabolism and regulate vital chemical processes in the body. Furthermore, proteins serve as messengers, enabling communication between cells and tissues. For example, hormones are proteins that transmit important signals throughout the body and control a wide range of physiological responses.
In addition to their structural, enzymatic, and signaling roles, proteins also contribute to immunity by acting as antibodies, protecting the body from diseases and infections. They recognize and bind to foreign substances such as bacteria and viruses, marking them for destruction by the immune system.
Proteins account for up to 40% of the volume of the cytoplasm. So far, there is no exact figure for how many different proteins the human body can produce. Scientists estimate that there are about 20.000 different proteins in the human body, but some studies suggest that there could be even more. The following schematic representations of different proteins are taken from an animation by BioVisions at Harvard University. A real image of a protein can be seen here.
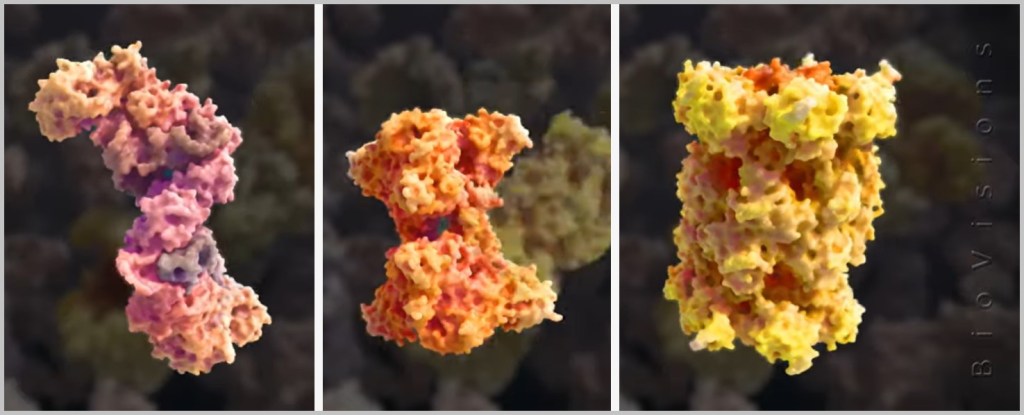
But how and where are these all-rounders created?
4. From Code to Protein – Cellular Mechanisms
4.1. From Signals to Actions
4.2. The Protein Biosynthesis
4.2.1. Transcription
4.2.2. Translation
4.3. The Protein
4.1. From Signals to Actions
Amid the bustling activity within the cell, crucial processes take place that sustain and enable life. One such process is protein synthesis, the building blocks of life. This complex task requires precise coordination and involves a series of steps that occur harmoniously within the cell. These cellular processes are regulated and orchestrated by the cell’s regulatory system.
The regulatory system detects external signals or changes within the cell to determine when there is a need for specific proteins. To produce the required proteins, the regulatory system activates so-called transcription factors. These transcription factors can be viewed as a type of production assistants, of which there is a great variety within the cell – estimated to be in the thousands. Each of them has specific permissions and a precise task to carry out. Transcription factors themselves are regulatory proteins.
Activated specific transcription factors now make their way to the nucleus, the location where the book of life – DNA – is found. This book contains all the information about the proteins that the cell can produce, encoded in thousands of genes. Therefore, it is a very precious book because without the book of life, the cell does not know how to produce the proteins. And without proteins, the cell cannot exist. The book of life is kept in a secure place, the nucleus, to ensure it is not damaged. You can imagine this secure location as a vault that can only be accessed with a valid access code.
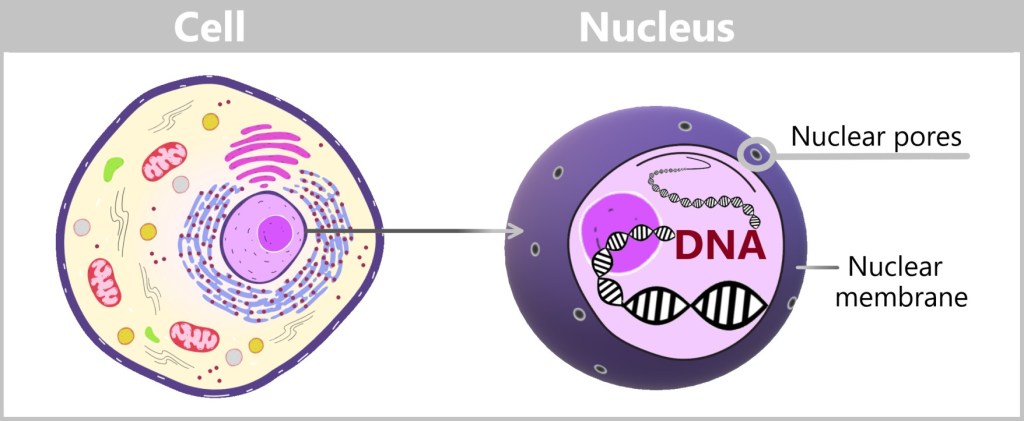
The nuclear membrane is the security barrier that surrounds the nucleus. It contains tiny nuclear pores that act as gatekeepers. This allows the nucleus to control who can enter or exit. Only those who can identify themselves and present the appropriate recognition signal are allowed to pass through the nuclear pores. [„Rush hour” at the nucleus, „How wriggling tentacles protect our genetic material.”]

The activated transcription factors can pass through the nuclear pores and carry out their tasks in the nucleus. There, they specifically search for particular DNA sequences near the starting point of the target gene, known as the promoter. These DNA sequences serve as anchor points where the transcription factors can bind to activate (or suppress) the transcription of the corresponding genes. By binding the transcription factors to these specific sites, RNA polymerase is attracted, thereby initiating the process of protein biosynthesis.
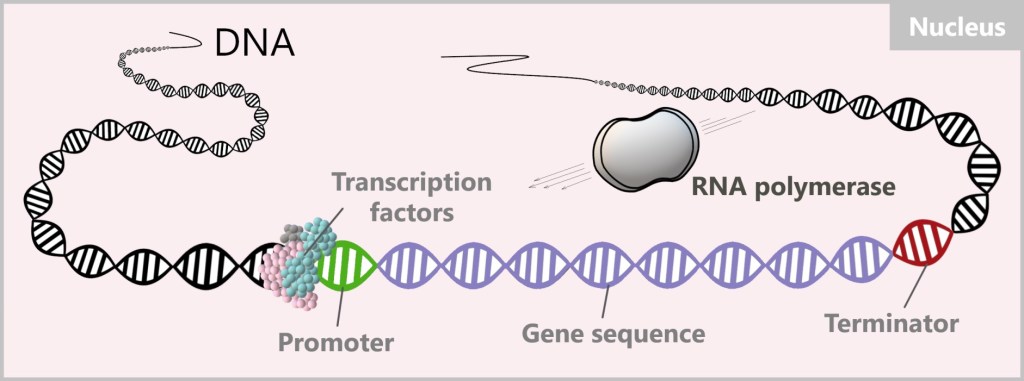
4.2. The Protein Biosynthesis
The process of protein production is called protein biosynthesis and it takes place in two sub-steps, transcription and translation.

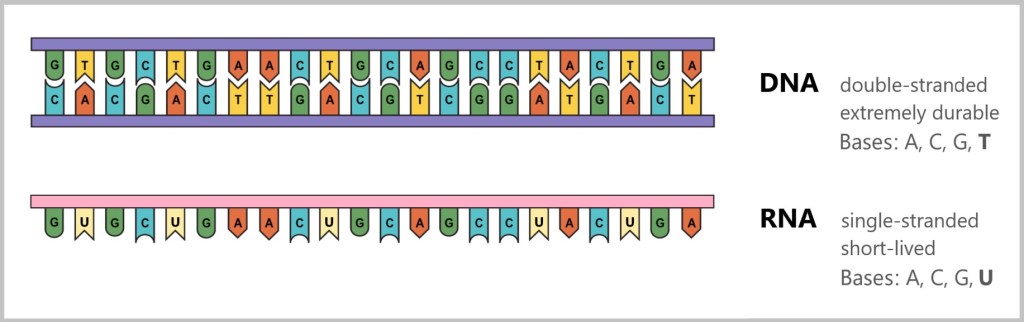
Difference between DNA and RNA
The difference between DNA and RNA is crucial for understanding how life functions at the molecular level.
DNA
DNA stands for deoxyribonucleic acid and is the molecule that contains all the genetic information of an organism. You can think of DNA as a recipe book containing all the instructions a cell needs to produce proteins and thus fulfil various tasks in the body.
Important Properties of DNA:
Structure: DNA has a double helix structure, i.e. it consists of two long strands that look like a twisted ladder.
Base Pairs: DNA is composed of four building blocks known as bases: adenine (A), thymine (T), guanine (G), and cytosine (C). The bases always form specific pairs: adenine (A) pairs with thymine (T), and guanine (G) pairs with cytosine (C).
Function: DNA stores all the instructions that a cell needs in the long term. It is stable and hardly changes.
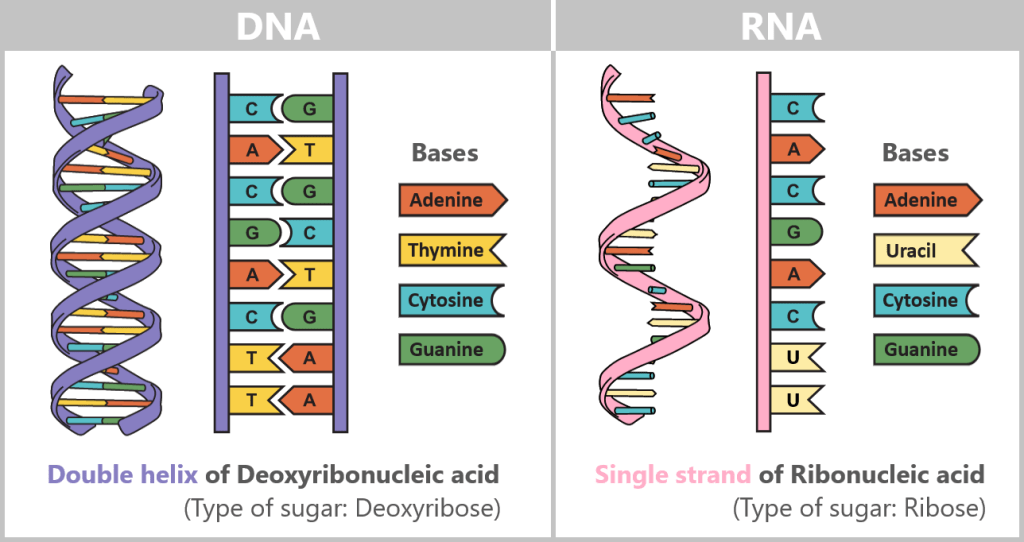
RNA
RNA stands for ribonucleic acid and has a structure similar to DNA, but it has some important differences. RNA is used as a „working copy” of DNA when a specific gene needs to be read and translated.
Important Differences to DNA:
Structure: RNA is usually a single strand, while DNA consists of two strands.
Bases: RNA uses almost the same building blocks as DNA, but there is one small difference: instead of thymine (T), RNA contains the base uracil (U).
Sugar: The sugar found in RNA is ribose, while DNA contains deoxyribose. This small difference makes RNA somewhat less stable and more short-lived than DNA.
Why is there mRNA?
mRNA stands for messenger RNA. It is essentially a copy of a specific section of DNA and is needed to convey the instructions from DNA for protein synthesis. Here are the reasons why mRNA is necessary:
DNA protection: The DNA remains in the nucleus because it is very valuable and sensitive. It is not used directly to produce proteins. Instead, a copy is made in the form of mRNA, which is then transported to the cell’s „factories” (the ribosomes) to produce proteins.
Working copy: The mRNA is a temporary copy of a gene. It is degraded after fulfilling its purpose. This makes RNA perfectly suited for short-term tasks, while DNA remains permanent and unchanged in the nucleus.
Why does the mRNA differ in one base (uracil instead of thymine)?
The transition from thymine (T) in DNA to uracil (U) in RNA is evolutionarily driven and is related to the function of the two molecules:
Stability: Thymine is chemically more stable than uracil, which is why it is found in DNA. DNA must remain stable throughout the life of an organism in order to preserve the genetic information.
Efficiency: Uracil is somewhat easier to produce than thymine and is completely sufficient for the short-term tasks of RNA. Since RNA exists only for a short time and is quickly degraded, it does not need to be as stable as DNA. Therefore, uracil is a more energy-efficient solution for RNA.
This division of labour means that the DNA remains protected and unchanged, while the RNA takes over the necessary tasks as a flexible working copy.
4.2.1. Transcription
The process of converting DNA into RNA is called transcription. An enzyme, RNA polymerase, is responsible for the production of RNA. An enzyme is a protein that accelerates biochemical reactions in the body.
Once the transcription factors have bound to the promoter, they act as a platform or anchor point for the RNA polymerase. It places itself on the transcription factors or in their immediate vicinity and initiates the process.
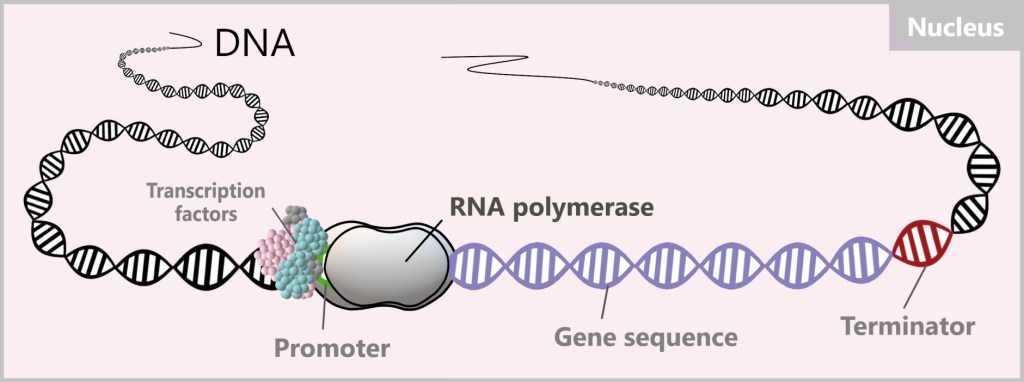
This begins with the unwinding of the DNA double helix, making the DNA sequence accessible. RNA polymerase moves along the DNA sequence and reads it.

During this process, RNA polymerase synthesizes an RNA strand using RNA nucleotides, the building blocks of RNA. The polymerase forms complementary base pairs between the RNA nucleotides and the bases of the DNA sequence. In this pairing, adenine (A) pairs with uracil (U), and cytosine (C) pairs with guanine (G). The difference from DNA is that in the RNA copy, the base thymine (T) is replaced by the base uracil (U), which is a characteristic feature of RNA.
Once a reading step is completed, RNA polymerase closes the DNA behind it. The reading process ends at the terminator, the section at the end of the target gene. The produced RNA and the polymerase then detach from the DNA. In this process, the DNA, the „precious book of life”, remains unchanged and undamaged.
Why is synthesis always in the 5′-3′ direction?
DNA consists of two strands that run in opposite directions. One strand runs in the 5′ to 3′ direction, while the other runs in the 3′ to 5′ direction. These two strands are complementary to each other.
The RNA polymerase binds to the template strand (mold strand), which runs in the 3′-5′ direction, and reads it in that direction. Meanwhile, it synthesizes a new RNA strand in the 5′-3′ direction by adding new RNA nucleotides to the 3′ end of the growing strand.
What do 5‘ and 3’ mean?
To understand the terms 5‘ and 3’, you need to look at the structure of the sugar in a nucleotide (see figure below). Nucleotides are the chemical building blocks of DNA, each consisting of a base (A,C,G or T), a sugar (Z) and a phosphate group (P). The sugar in DNA is deoxyribose, a molecule with five carbon atoms (C), which are numbered as follows:
1′: Here, the base (A, T, C, or G) is attached.
2′: It carries only a hydrogen atom (-H) in DNA and no hydroxyl group (-OH) as in RNA. This missing OH group gives deoxyribose its name (‚deoxy‘-ribose, since ‚deoxy‘ means without oxygen).
3′: A hydroxyl group (-OH) is located here, which is important for the addition of new nucleotides during synthesis.
4′: It connects the ring of the sugar molecule to the 5′ carbon.
5′: Carries the phosphate group that links the nucleotide to the next one.
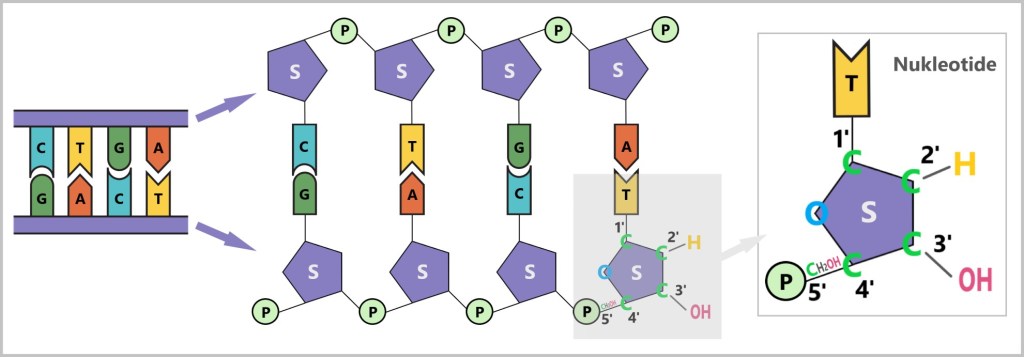
(A,C,G,T – bases, S – sugar, P – phosphate group, C – carbon atom)
Why does the synthesis only take place in the 5‘-3’ direction?
The RNA polymerase can only add new nucleotides to the 3′ end of the growing strand because a hydroxyl group (-OH) is present there. This OH group enables the chemical reaction in which the phosphate group of the new nucleotide is bound to the strand. As the 5‘ position of the new nucleotide is already occupied by a phosphate, the chain extension can only take place in the 5’-3′ direction.
How does reading and synthesizing work?
The RNA polymerase moves along the template strand of the DNA in the 3‘-5’ direction. As it does so, it adds complementary RNA nucleotides to the new RNA strand, which grows in the 5‘-3’ direction. The new nucleotides are always attached to the free 3′-OH group of the growing strand.
Detailed information on this topic can be found here and here.
The resulting RNA is a copy of the original DNA code.
The RNA that is directly produced from transcription is called pre-mRNA and must undergo further processing steps to become mature mRNA, which is ready for translation. This processing includes splicing, where introns (non-coding regions of a gene) are removed and exons (coding regions of a gene) are joined together.
Introns and exons are not constant but variable, and they play an important role in gene regulation and diversity. This means that depending on the type of protein that needs to be produced, different exons can be combined and introns removed. Through this process, cells can produce a variety of proteins that perform different functions.
Additionally, a 5′-cap is added to the 5′-end of the mRNA, and a poly-A tail (a series of adenine nucleotides) is added to the 3′-end.
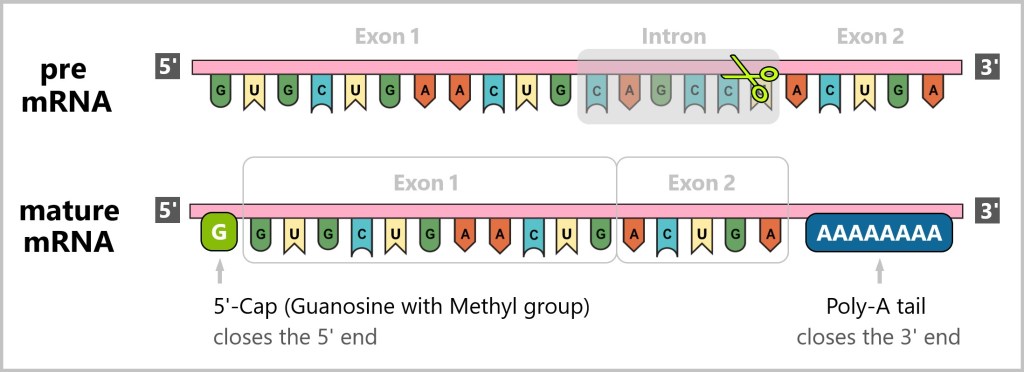
The addition of the 5′-cap and the poly-A tail to mRNA serves important functions.
The 5′-cap and the poly-A tail ensure the stability of the mRNA, enable its safe transport and ensure that it is efficiently utilised for protein production.
5′-Cap
Protection against degradation: The 5‘-cap is a modified guanine base that is attached to the 5’-end of the mRNA. It protects the mRNA from degradation. Without this protection, the mRNA could be rapidly degraded before it reaches translation.
Facilitation of transport: The 5′-cap also assists in the transport of mRNA from the nucleus to the cytoplasm. It is recognized by specific transport proteins that guide the mRNA through the nuclear pores into the cytoplasm.
Initiation of translation: The 5′-cap is crucial for the recognition and binding of mRNA to the ribosomal subunit, which is necessary for the initiation of translation. Without the cap, the mRNA cannot be efficiently recognized by the ribosome.
Poly-A-tail
Stability of the mRNA: The poly-A tail consists of a series of adenine nucleotides attached to the 3′ end of the mRNA It protects the mRNA from degradation by enzymes that break down RNA from its 3′ end. Longer poly-A tails extend the lifespan of the mRNA in the cytoplasm, leading to more efficient protein production.
Regulation of Translation: The poly-A tail also contributes to the regulation of translation. It controls the frequency of mRNA translation by influencing the interaction with ribosomes and other proteins necessary for translation.
Stability during Translation: The poly-A tail can help maintain the mRNA in a specific spatial conformation that is necessary for efficient translation.
The mature mRNA, which contains the genetic blueprint for the protein to be produced, diffuses through the nuclear pores into the cytoplasm of the cell, where it is recognized by the ribosomes. This initiates the next step – translation.

Biotech Made Easy offers a short visualisation of the transcription process, which makes the process easy to understand.
Another short animation of the transcription comes from the DNA Learning Centre. It shows the process in real time.
4.2.2. Translation
Translation is the process by which the genetic information stored in the mRNA is converted into a sequence of linked amino acids to form proteins. This important transformation takes place at the ribosomes, the protein factories of the cell. The ribosomes act as machines that read the mRNA and arrange the amino acids according to the instructions of the mRNA.
In eukaryotes like humans, two types of ribosomes are distinguished: free ribosomes and bound ribosomes (see Fig. 15). A significant portion of the ribosomes is found freely in the cytoplasm, while others are bound to the membrane of the endoplasmic reticulum (ER). The ribosomes attached to the ER produce proteins that are either intended for export from the cell or for incorporation into the cell membrane. Both free and membrane-bound ribosomes have a similar structure and function. For our further consideration, we will focus on the free ribosomes.
The ribosome binds to the mRNA and then moves along the mRNA sequence from the 5′ end to the 3′ end. During this process, the mRNA slides through the ribosome like a conveyor belt and is scanned step by step. Three consecutive bases, known as codons, are read at a time. When the ribosome reaches the start codon (AUG), translation begins.
During translation, the ribosome reads each codon on the mRNA and assigns it a corresponding transfer RNA (tRNA) with the appropriate amino acid. The tRNA carries a specific sequence of three nucleotides, known as an anticodon, which is complementary to the codon of the mRNA.

When the anticodon of a tRNA pairs with the corresponding codon of the mRNA, the amino acid carried by that tRNA is linked to the growing peptide chain. Peptides are short chains of amino acids, and these amino acids are the building blocks of proteins. This process repeats as the ribosome moves along the mRNA, scanning each codon.
The amino acids that are linked together are connected by peptide bonds, forming the peptide chain of the emerging protein. Translation ends when the ribosome reaches a stop codon (UAA, UAG, or UGA) on the mRNA. At this point, the newly formed peptide chain – the unfinished protein – is released, and the ribosome detaches from the mRNA.
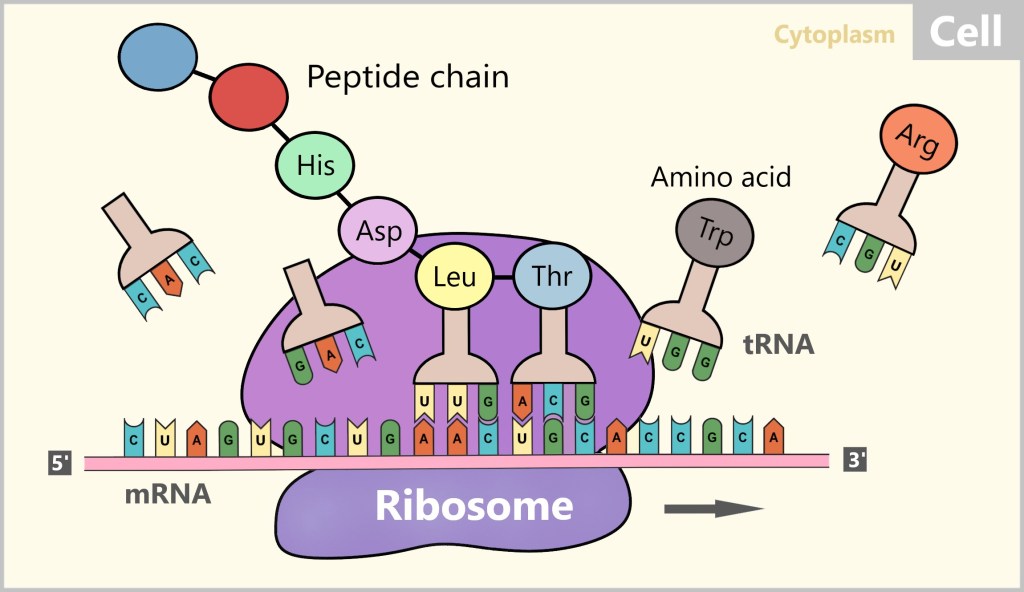
Here, too, there is a brief and clear visualisation of the Biotech Made Easy translation process.
4.3. The Protein
The protein initially exists as a chain of a specific sequence of amino acids. For the newly synthesized protein to become functional, it must fold into the correct three-dimensional shape. The spatial arrangement of the amino acid chain – its specific three-dimensional form – is crucial for the protein to fulfill its role in the body, whether as an enzyme (a molecule that accelerates chemical reactions), a structural protein (which gives cells and tissues their shape and strength), or a hormone (a signaling molecule that regulates various bodily functions).
Every protein has its own blueprint that determines how it will fold to fulfill its function. The amino acid chain forms the primary structure, from which repeating patterns arise, referred to as the secondary structure. Electrostatic attractions and other chemical bonds then bring different parts of the protein together, resulting in its final shape – the tertiary structure. Sometimes, multiple proteins even form complex structures known as quaternary structures.
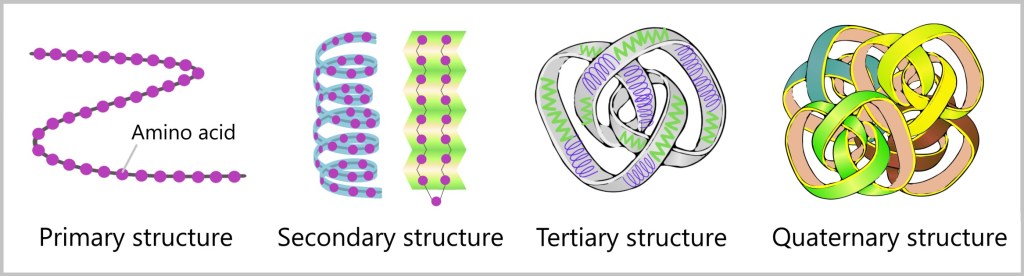
The path to proper folding is not always straightforward. Sometimes proteins can misfold, especially when they are too close to each other. In these moments, helpers come into play – the chaperones, which are proteins themselves. They protect the peptide chain from unwanted interactions and facilitate the folding process.
However, some proteins are too large to fold on their own. Here again, chaperones come into play, guiding the proteins to another helper – a cylindrical structure called a chaperonin. In this secure environment, proteins can fold undisturbed. The fully folded proteins are now ready to begin their work inside or outside the cell.
The video „Chaperones – Protein Folding” illustrates the process of protein folding in an engaging way.
Errors in protein folding
The process of protein folding is crucial for the biological functionality of the cell and is carefully regulated. A misfolded protein can lose its normal function or even become harmful. Errors in protein folding can have serious impacts on cellular processes and lead to various diseases. Examples include Amyloidosis, Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease.
The „Protein Folding Problem”
The „protein folding problem” is one of the central puzzles of biology. It refers to the difficulty of predicting the three-dimensional structure of a protein based on the sequence of its amino acids. Proteins are made up of hundreds of amino acids that fold into complex spatial structures, and the DNA sequence alone does not provide direct information about this structure. The challenge of protein structure prediction lies in inferring the spatial arrangement of the amino acids from the genetic information. The magnitude of this challenge becomes apparent when considering that the number of theoretical possibilities for how a protein could fold before achieving its final 3D structure is enormous.
Scientists from various disciplines have been working on solving this puzzle for more than 50 years. A groundbreaking development in this field is the creation of ‚AlphaFold‚ by Deepmind, an AI that handles protein structure prediction with unprecedented accuracy. This achievement could revolutionise the understanding of protein folding.
Nevertheless, the exact process of protein folding is still not fully understood and remains a current area of research in biochemistry.
Proteins: Their Lifespan and Regeneration
Like any product, a protein has a limited lifespan. Once a protein has fulfilled its function or is damaged, it is broken down. This continuous process occurs in all cells of the body.
A specialized enzyme called the proteasome is involved in this process. The proteasome cuts the protein into smaller fragments (peptides), which are then further broken down into individual amino acids. These amino acids can be reused to produce new proteins. This ensures that faulty or no longer required proteins are removed and the cell’s resources are utilised efficiently.
Protein degradation and its connection to the immune system
The degradation of proteins is an essential process that has far-reaching effects on various systems in the body, including the immune system. The specific role this process plays in the immune system will be discussed in more detail in the following chapters.
5. The Body’s Shield – Our Immune System
Our immune system is the impressive legacy of evolution. It is a fascinating micro-world that works in secret – an army of immune cells that have developed into masterpieces of adaptation over millions of years. These tiny fighters stand ready day and night to protect us from the countless threats that we often don’t even realise are there. They watch over us while we sleep, fight for us without us realising it, and are ultimately the reason why we can live and breathe.
To ensure survival, all higher organisms have developed an immune system that fends off pathogens, prevents infections, or contains them. The immune system is composed of a variety of cells, tissues, and organs that work together to protect the body.
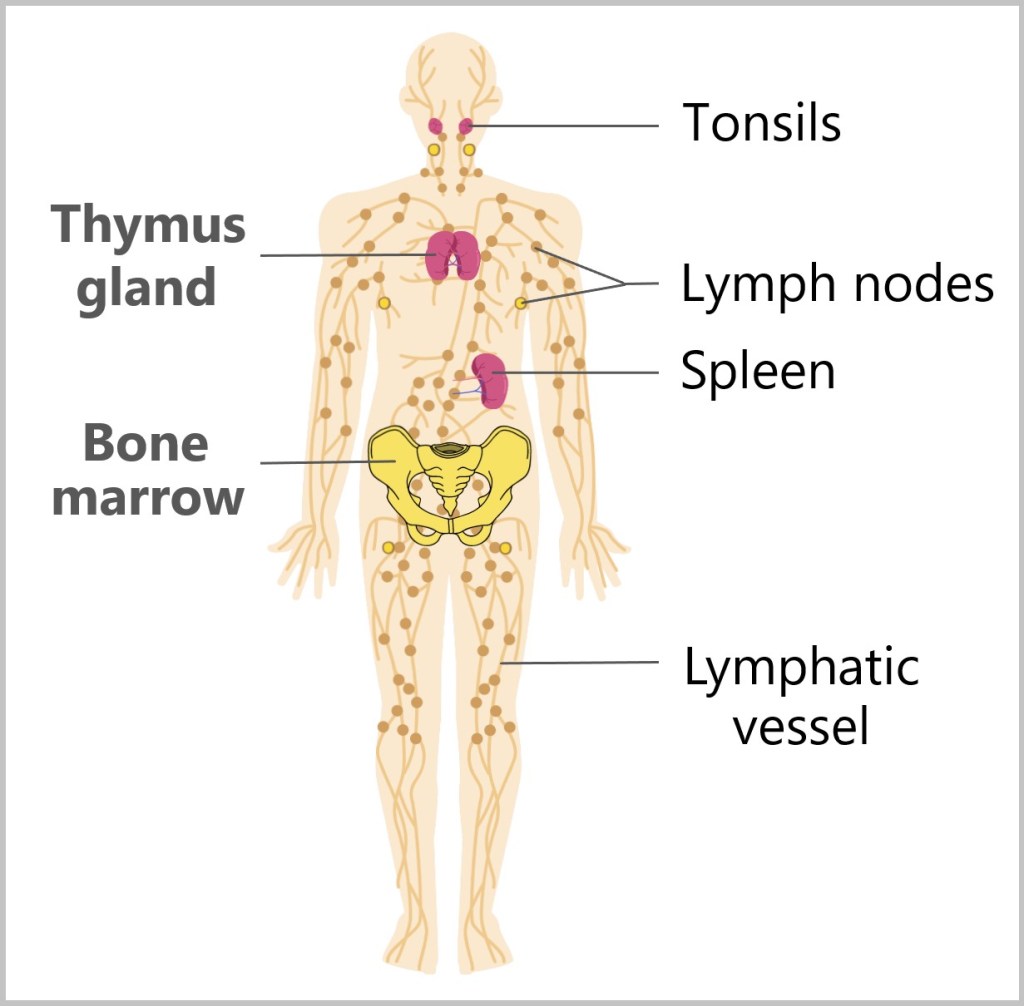
The key components include white blood cells (leukocytes), which come in various forms and have different functions, as well as lymphatic organs such as the bone marrow, thymus gland, spleen, tonsils, and lymph nodes.
Fig. 19: Components of the immune system
In addition, the immune system has its own transport network, the lymphatic system, which carries lymph (a watery, slightly milky fluid) and immune cells through lymphatic vessels across the body, enabling them to quickly reach and combat infection sites.
The interaction between immune cells is highly complex and will be simplified here. Although this presentation is already quite challenging, the actual processes in the body are even more intricate and not yet fully understood. To make this easier to grasp, we will focus on the key immune cells and their most important processes.
5.1. Origin of Immune Cells
5.2. Mechanisms of Immune Recognition: SELF vs. NON-SELF
5.3. The Nonspecific Immune Defense
5.4. The Complement System
5.5. The Specific Immune Defense
5.6. Summary

The basic principle of the immune system is based on distinguishing between NON-SELF and SELF, a mechanism that is present from birth.
To defend itself against invaders, the body must be able to differentiate between foreign microorganisms and its own tissue. Not everything foreign is dangerous, so the immune system also needs to distinguish between harmless foreign substances (such as dust, pollen, or food) and harmful ones. Anything that causes illness is considered dangerous to the body, meaning it is pathogenic. The term pathogens encompasses all foreign entities that can cause disease. Pathogens include bacteria, viruses, fungi, and parasites.
The immune system recognizes so-called antigens based on the structures on the surface of pathogens or other foreign substances. The term ‚antigen‘ means ‚antibody generating‘. These antigens are typically proteins or polysaccharides and serve as „recognition markers” that signal to the immune system that a foreign body is present.
The immune system’s response to anything NONSELF is an immune response. It involves a variety of processes aimed at eliminating the threat of foreign substances and maintaining the integrity of the body.
The right balance between tolerance and defence reaction as well as the strength of the reaction are a real challenge for the immune system. This only works if the individual components of the immune system work together harmoniously.
Throughout evolution, coordinated defense systems have developed to work together. In general, there is a distinction between the innate (nonspecific) and adaptive (specific) immune defenses. The innate immune system is the body’s first line of defense against pathogens. Specialized immune cells respond to threats based on a pre-programmed response. In contrast, the adaptive immune system is highly specialized and targets specific pathogens with precision, recognizing and eliminating them in a targeted manner.
Before we dive deeper into the immune defense, let’s take a look at the origin of immune cells and the mechanisms the immune system uses to differentiate between foreign and self-cells. In this context, key terms and concepts will be introduced, which will be repeatedly referenced throughout the discussion.
5.1. Origin of Immune Cells
Blood cells and immune cells originate from hematopoietic stem cells in the bone marrow. These stem cells are the common source for both blood cells and immune cells, as immune cells are an essential part of the blood. The differentiation of these stem cells follows either the myeloid or lymphoid lineage, leading to the development of various cells involved in both the innate (nonspecific) and adaptive (specific) immune systems.
In adults, blood formation (hematopoiesis) primarily occurs in the bones of the pelvis, sternum, ribs, and parts of the spine. These bones contain red bone marrow, which houses the stem cells that give rise to both blood and immune cells. In children, blood formation also takes place in the long bones of the arms and legs, such as the femur and humerus. As individuals age, this bone marrow is gradually replaced by fat-rich yellow bone marrow, causing blood formation to become mainly concentrated in the pelvis and spine.

As shown in the illustration, immune cells develop from two main lineages:
Myeloid Lineage
This line leads to the formation of cells of the innate immune system, including:
- Neutrophil granulocytes
- Eosinophil granulocytes
- Basophil granulocytes
- Mast cells
- Monocytes (which develop into macrophages and dendritic cells)
Lymphoid Lineage
This line forms the cells of the adaptive immune system:
- T lymphocytes
- B lymphocytes
Natural killer cells also originate from the lymphoid lineage but are part of the innate immune system.
Our blood
On average, we adults have between 5 and 6 litres of blood in us. Blood is a fluid tissue that fulfils a variety of vital functions in the body. It consists of a liquid part, the blood plasma, and three solid components, the blood cells. Blood cells make up around 45 per cent of the total blood volume. There are three main types of blood cells: erythrocytes, thrombocytes and leucocytes. Each type of blood cell has specific functions.

Erythrocytes (red blood cells)
Function: Transport of oxygen from the lungs to the tissues and return transport of carbon dioxide to the lungs.
Proportion: Approximately 45% of the total blood volume (hematocrit).
Quantity: Approximately 4.5 to 5.9 million cells per microliter of blood.
Size: About 6-8 micrometres in diameter.
Lifespan: About 120 days.
Characteristics: Erythrocytes have no nucleus, which allows for more space for hemoglobin. The absence of a nucleus means that they cannot divide or synthesize proteins. Their unique structure and the properties of their cell membrane enable them to withstand the mechanical stresses of the bloodstream for a long time.
Thrombocytes (platelets)
Function: Important for blood clotting and wound closure. They form blood clots to stop bleeding.
Proportion: Less than 1% of the total blood volume.
Quantity: Approximately 150.000 to 450.000 platelets per microlitre of blood.
Size: About 2-3 micrometres in diameter.
Lifespan: 7-10 days.
Leukocytes (white blood cells)
Function: Combatting germs and pathogens, as well as forming antibodies.
Proportion: Less than 1% of the total blood volume.
Quantity: About 4.000 to 11.000 cells per microlitre of blood.
Subgroups:
Neutrophil granulocytes: 50-70% of leucocytes; part of the innate immune system and important for fighting bacterial infections. Lifespan: 5-90 hours in blood; 1-2 days in tissue. Size: 10-12 micrometres in diameter.
Eosinophil granulocytes: 1-4% of leucocytes; part of the innate immune system and important in combating parasites and allergic reactions. Lifespan: 8-12 hours in blood; 8-12 days in tissue. Size: 12-17 micrometres in diameter.
Basophil granulocytes: <1% of leukocytes; part of the innate immune system and involved in allergic reactions and the release of histamine. Lifespan: 1-2 days in the blood. Size: 10-14 micrometres in diameter.
Lymphocytes: 20-40% of leucocytes; play a key role in the adaptive immune response. Lifespan: A few days to several years. Size: 6-10 micrometres in diameter for small lymphocytes and up to 15 micrometres for larger lymphocytes.
Natural killer cells: 5-15% of lymphocytes; part of the innate immune system, responsible for killing virus-infected cells and tumour cells. Lifespan: Weeks to months. Size: 10-15 micrometres in diameter.
Monocytes: 2-8% of leucocytes; precursor of macrophages and dendritic cells. Lifespan: 1-3 days in the blood; weeks to months in the tissue as macrophages or dendritic cells. Size: 15-20 micrometres in diameter.
Mast cells: Although they do not circulate in the blood, mast cells in tissues (e.g. skin, mucous membranes) are important for the defence against parasites and play a key role in allergic reactions by releasing histamine and other mediators. Lifespan: Weeks to months in the tissue. Size: 10-16 micrometres in diameter.
Blood plasma
Proportion: About 55% of the total blood volume.
Composition: Primarily composed of water (approximately 90 percent).
Function: Transport of nutrients, waste products and hormones.
5.2. Mechanisms of Immune Recognition:
SELF vs. NON-SELF
The distinction between SELF and NON-SELF is primarily made by specialized immune cells that specifically scan the surfaces of cells – both those of the body and of pathogens. Since cells neither have eyes nor ears, they must make contact to determine whether a protein belongs to a friend or foe. For this purpose, they are equipped with a variety of receptors.
Body cells carry so-called SELF markers on their surface. These markers are specific molecules or receptors that are recognized by the immune system and are typically not attacked.
Pathogens, on the other hand, possess specific structures on their surface that are not present on the body’s own cells. Similarly, mutated or damaged cells reveal themselves through changes on their surface. These structures can be interpreted as NON-SELF markers, prompting the immune system to trigger a defensive response.
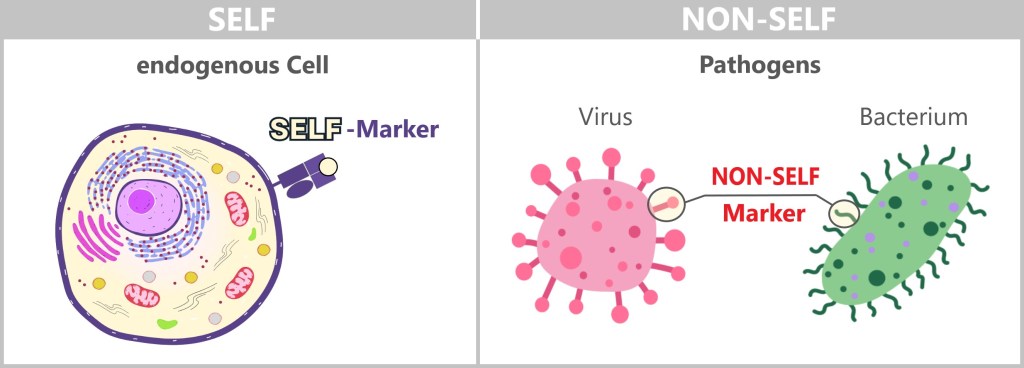
But how do immune cells communicate with each other to ensure a coordinated response to threats? The answer lies in signaling molecules called cytokines and chemokines.
Cytokines are small proteins that act like signaling beacons, controlling the behavior of immune cells. They regulate the growth, activation, and function of immune cells and play a crucial role in managing inflammatory responses. Depending on the situation, they can either amplify or dampen the immune response.
Chemokines, on the other hand, direct the movement of immune cells throughout the body. They act as navigators, guiding immune cells to the site of infection or inflammation. In this way, they ensure that the body’s defenses are activated precisely where they are needed.
SELF-Marker
SELF-markers, also known as autoantigens, serve as identifiers for the body’s own tissues. During its development, the immune system learns to recognize and tolerate these markers in order to avoid autoimmune reactions. The immune system has several mechanisms in place to achieve this, with three important ones highlighted here:
a) SELF-Marker: MHC Molecules
b) SELF-Marker: CD47 Molecule
c) SELF-Marker: Sialic Acid
a) SELF-Marker: MHC Molecules
An important example of SELF-markers are MHC molecules. The term MHC stands for Major Histocompatibility Complex and refers to special proteins on the cell surface that function as receptors. These receptors primarily present the body’s own protein fragments. By doing so, the cell signals to the immune system that it belongs to the body and does not pose a threat.
However, MHC molecules can also present foreign protein fragments. These usually come from pathogens or diseased cells, such as cells infected by viruses. By displaying such foreign fragments on the cell surface, the immune system can recognize infected or abnormal cells and initiate an appropriate defense response.
Major indicates the significant importance of these genes for immune recognition.
Histocompatibility is composed of ‚histo‘ (tissue) and ‚compatibility‘, referring to how well tissues are compatible or acceptable between different individuals. MHC plays a crucial role in organ transplantation.
Complex refers to the group of genes that work together to perform a complex function.
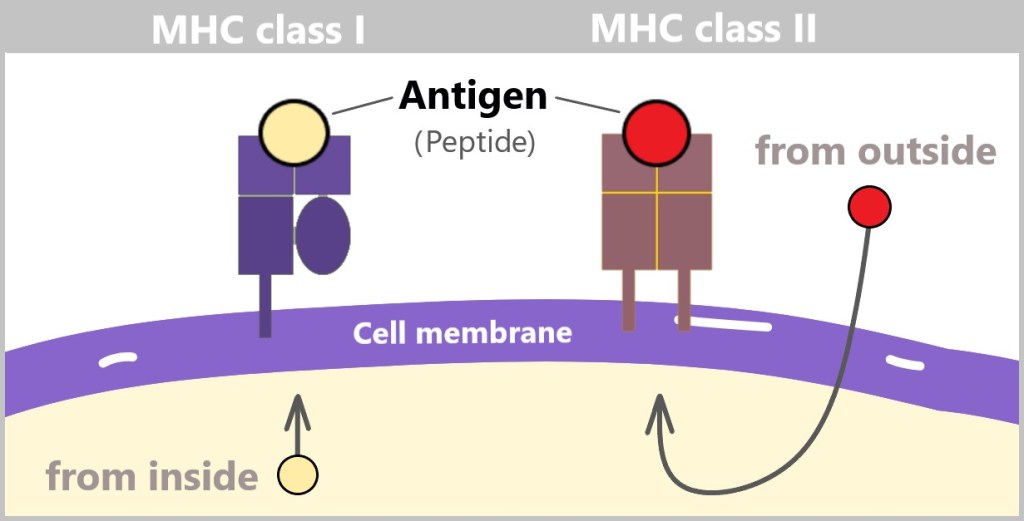
There are two main classes of MHC molecules:
MHC class I molecules are found on almost all nucleated cells in the body.
MHC class II molecules are mainly found on specific immune cells.
The following simplified graphic illustrates the difference between the two classes. Both MHC class I and MHC class II molecules are anchored in the cell membrane and present protein fragments (peptides) that serve as antigens. A structural difference is that MHC class I molecules have a single anchoring point in the cell membrane, while MHC class II molecules have two anchoring points.
In general, MHC complexes serve the purpose of antigen presentation by providing the immune system with information about the state of the cell.
Antigen presentation
MHC-I presents antigens that originate from inside the cell. As mentioned in section ‚4.3. The protein‘, cells continuously degrade old or damaged proteins. In the process, proteasomes break down these proteins into smaller fragments, so-called peptides. These peptides can originate from the body’s own proteins and signal to the immune system that the cell is healthy. However, if the cell is infected with a virus or otherwise becomes abnormal, the MHC-I molecule presents peptides from foreign or altered proteins. This alerts the immune system that the cell is potentially dangerous and needs to be eliminated.
MHC class I molecules are expressed by all nucleated cells.

On the left is a healthy cell presenting its own normal peptide via an MHC class I molecule. This indicates that the cell is healthy and not infected. The MHC-I molecule signals to the immune system that no action is needed. In the middle is a virus-infected cell, which also carries an MHC class I molecule but presents a foreign peptide derived from a virus. This viral peptide alerts the immune system that the cell is infected, potentially triggering an immune response. On the right is a cancer cell that presents a modified peptide through an MHC class I molecule. This peptide has been altered due to genetic mutations occurring during cancer development. It signals to the immune system that the cell is abnormal and should be removed.
MHC class II, on the other hand, presents antigens that originate from outside the cell. MHC-II molecules are expressed only by specialized immune cells, such as macrophages, dendritic cells, and B cells. These cells engulf foreign material, such as bacteria or other pathogens, break it down into smaller fragments (antigens), and then present these on their cell surface. The antigens presented in this way activate specific immune cells that coordinate a targeted immune response.
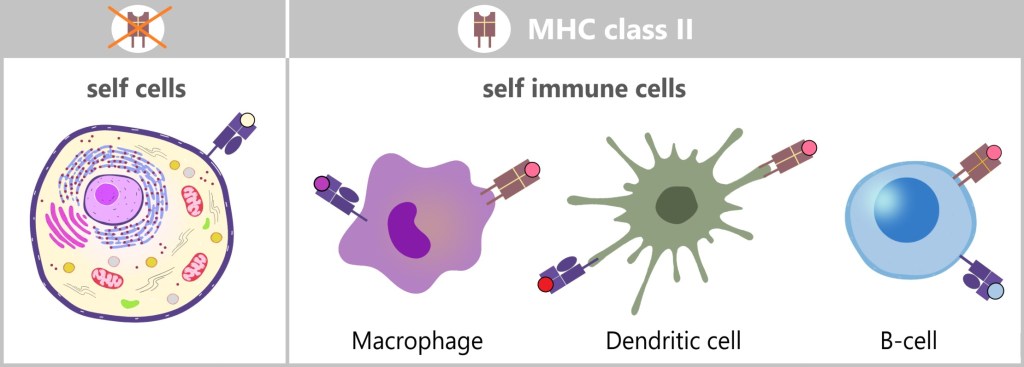
MHC molecules enable the immune system to differentiate between the body’s own cells and foreign or altered cells. This is crucial for protecting healthy cells and at the same time triggering an immune response against potentially harmful invaders or abnormal cells.
The short animation on antigen presentation: MHC class I vs. MHC class II illustrates the relationships explained above.
MHC molecules and their diversity
What are MHC molecules?
MHC molecules are special proteins on the surface of our cells. They help the immune system to differentiate between our own and foreign substances, such as pathogens. MHC molecules are formed by certain genes in our body.
What are alleles?
The genes that produce MHC molecules come in many different versions, known as alleles. Each person has a unique combination of alleles, which causes their MHC molecules to appear slightly different from one individual to another.
Why are there so many versions?
There are hundreds of different alleles within the population. This vast diversity helps the immune system recognize a wider range of pathogens. As a result, some people are better protected against certain diseases than others – which is an advantage from an evolutionary perspective. [Major Histocompatibility Complex]
How do MHC molecules work?
Each MHC molecule can bind small fragments of proteins, so-called peptides. These peptides are produced when proteins are broken down in the body. MHC molecules then present these peptides on the cell surface so that the immune system can recognise whether the cell is healthy or has been attacked by a virus or other pathogens.
Why is this diversity important?
The diversity of MHC molecules means that different people can bind and present different peptides. This makes it more difficult for pathogens to infect all people in the same way, as each person’s immune system responds differently to them.
Organ transplantation and MHC molecules
The diversity of MHC molecules is also the reason why finding compatible organ donors is challenging. If the donor’s MHC molecules do not match well with those of the recipient, the recipient’s immune system may recognize the new organ as foreign and attack it. Therefore, in organ transplantation, the MHC molecules of the donor and recipient must be carefully compared to reduce the risk of rejection.
b) SELF-Marker: CD47 Molecule
Another important SELF-marker on the surface of cells is the molecule CD47. This molecule acts as a ‚Don’t eat me‘ signal, meaning it prevents immune cells from destroying the cell.
CD47 plays a crucial role in the regulation of the immune response and the protection of blood cells. In contrast to MHC class I molecules, which are only found on nucleated cells, CD47 is found on all cell types, including nucleus-free cells such as erythrocytes (red blood cells) and thrombocytes (platelets).
CD47 binds to a receptor protein called SIRPα (Signal Regulatory Protein Alpha), which is present on the surface of immune cells. This binding sends a signal that prevents the immune cell from attacking the cell. This ensures that healthy, self-cells are not destroyed by the immune system.
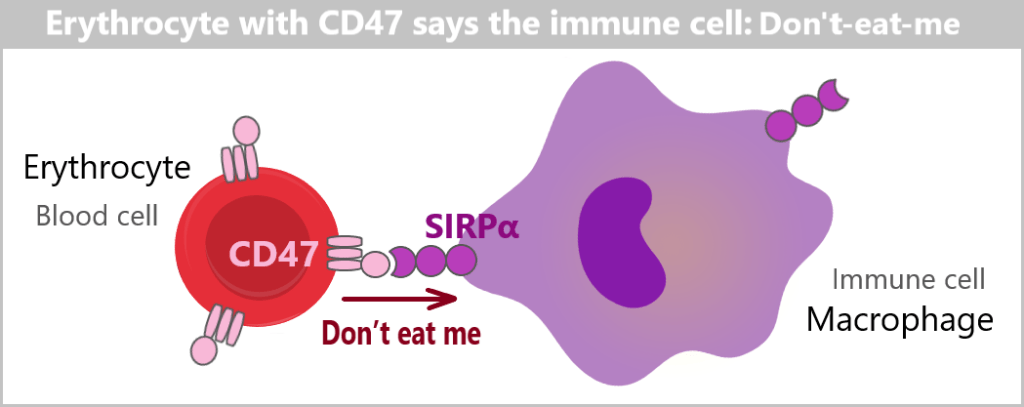
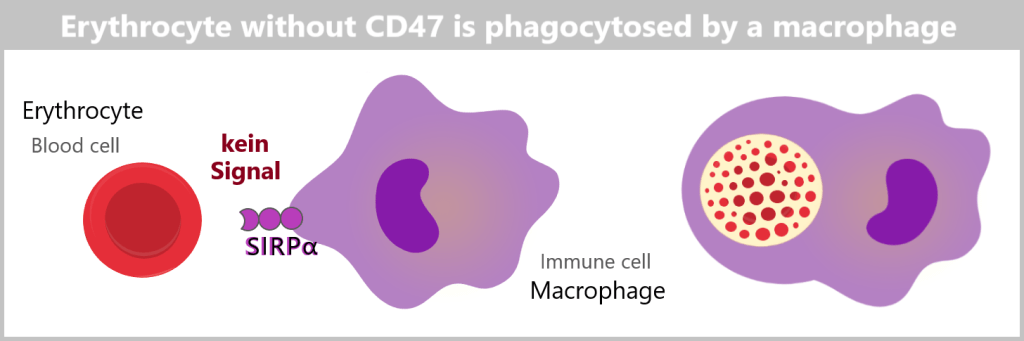
If the CD47 marker is missing or not functioning properly, the cell can be recognized as „foreign” or „altered”. This leads immune cells to attack and eliminate these cells.
CD47 molecules do not differ as much from person to person as MHC class I molecules do. They have a relatively conserved structure in most individuals, meaning there are fewer variations. This conservation is important because CD47 plays a fundamental role in immune regulation and cell interaction.
For this reason, a blood transfusion generally works more smoothly than an organ transplant. In a blood transfusion, only a few antigens (such as blood group characteristics) need to be considered, while in an organ transplant, there is a high variability in MHC class I molecules, which can trigger a strong immune response.
c) SELF-Marker: Sialic Acid
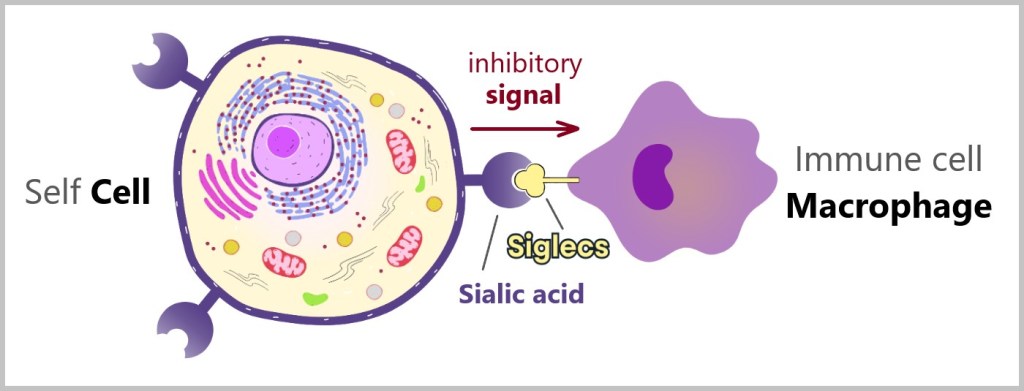
Another significant SELF-marker on the cell surface is sialic acid. Sialic acids are a group of sugar molecules found on the surfaces of cells and play an important role in cell-to-cell communication. All of the body’s own cells carry sialic acid on their surface.
Special receptors on the surface of immune cells can recognize and bind to sialic acids. These sialic acid-binding receptors are known as Siglecs (sialic acid-binding immunoglobulin-type lectins). When sialic acids bind to Siglecs, they send inhibitory signals into the immune cell, preventing it from attacking the cell. This mechanism protects the body’s own cells from attacks by the immune system.
Combination of these SELF-markers
A cell can carry MHC molecules, CD47, and sialic acid simultaneously on its surface. Each of these molecular groups serves an important function in marking the cell as self and protecting it from unnecessary immune responses. While MHC primarily serves the purpose of antigen presentation, CD47 and sialic acid ensure that cells are not attacked or eliminated by immune cells. Thus, these markers work together to efficiently regulate the immune system.
5.3. The Nonspecific Immune Defense
Our immune system continuously monitors the body for the presence of foreign substances and cell changes. As soon as something is recognized as a threat, the defense response is initiated. The nonspecific immune response is particularly fast, becoming fully activated within minutes to a few hours.
This form of immune defense is not specialized for specific pathogens. „Nonspecific” means that a general standard response occurs to every recognized threat; pathogens are fought in the same way regardless of their type. The nonspecific immune system is present at birth, which is why it is also referred to as the natural or innate immune system. In the first step, the organism attempts to prevent or at least hinder the invasion of pathogens.
I – First Line of Defense: Mechanical and Chemical Barriers
II – Second Line of Defense: White Blood Cells
5.3. a) Granulocytes
5.3. b) Macrophages
5.3. c) Dendritic Cells
5.3. d) Natural Killer Cells
I – First Line of Defense: Mechanical and Chemical Barriers
The first line of defense in our body against pathogens consists of mechanical and chemical barriers.
Mechanical barriers
Mechanical barriers include the skin and all mucous membranes. The skin, the boundary between the inside of the body and the outside environment, is our body’s most important defence against pathogens. It consists of several layers that prevent the penetration of pathogens. The outermost layer, the epidermis, renews itself regularly. Dead skin cells are constantly shed and replaced by new cells. This renewal process helps to remove microorganisms adhering to the skin.
Mucous membranes are found in many areas of our body, including the nose, mouth, throat, lungs and digestive tract. They produce mucus, a sticky substance that traps and removes pathogens. For example, the mucus in our nose traps dust and microorganisms that we breathe in, preventing them from entering our lungs.
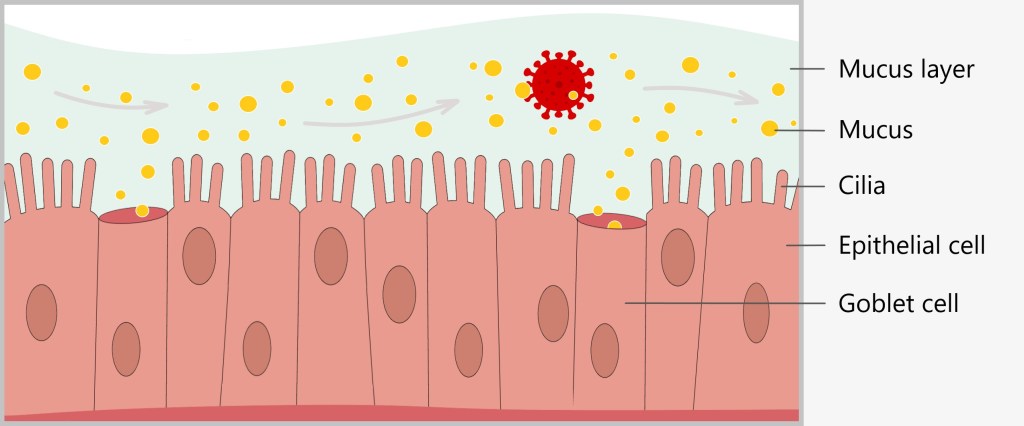
Mucosal cells have many cilia, which lie next to each other like a dense lawn. Goblet cells constantly produce mucus that surrounds the cilia and covers the surface of the mucous membrane. Foreign particles such as viruses, bacteria or dust easily become trapped in this viscous layer of mucus.
The cilia move in a wave-like manner to transport foreign particles trapped in the mucus out of the airways. Similar to a conveyor belt, the cilia push the mucus layer, along with the foreign bodies, towards the throat. There, the secretions can be expelled through sneezing or coughing, or swallowed. This self-cleaning mechanism is constantly active, ensuring that the respiratory tract remains clear of harmful substances and pathogens.
Chemical barriers
In addition, chemical substances such as acids, enzymes, or mucus hinder the attachment of pathogens. Certain areas of our body, such as the skin and the stomach, have an acidic environment. This acidic milieu is unfavorable for many microorganisms and can inhibit their growth or kill them. Particularly in the acidic environment of the stomach, many pathogens are rendered harmless.
Enzymes are proteins that catalyze chemical reactions. Some enzymes in our body, such as lysozyme found in our saliva and tears, can break down bacterial cell walls, thus killing them. As mentioned earlier, our mucous membranes produce mucus that traps pathogens. However, mucus also serves as a chemical barrier. It contains antimicrobial substances such as immunoglobulins, which can neutralize pathogens.
Although the mechanical and chemical barriers of our body are highly effective in preventing the entry of pathogens, some pathogens can still overcome these barriers. Once pathogens have breached these initial defenses, our body activates another line of defense.
II – Second Line of Defense: White Blood Cells
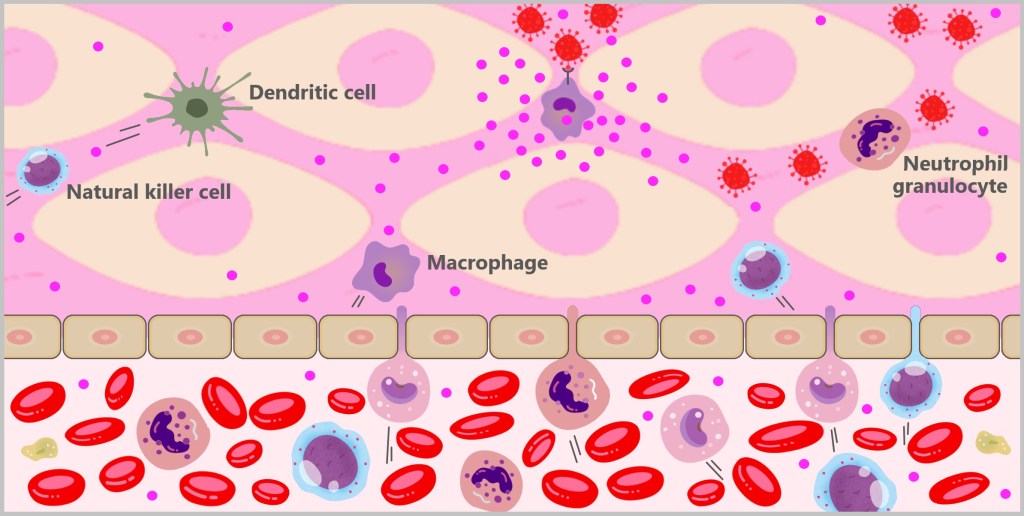
If the pathogens manage to overcome the first line of defence and enter the body, they do not go unnoticed for long. Specialised immune cells such as granulocytes, macrophages, dendritic cells and natural killer cells are ready to defend the body. These cells belong to the white blood cells, the leukocytes.
Granulocytes, monocytes, and natural killer cells circulate in the blood and migrate into tissues when needed. When monocytes enter the tissues, they develop into macrophages or dendritic cells, which then reside in the tissue and carry out their immune functions.

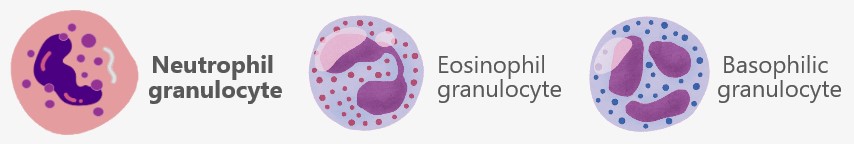
5.3. a) Granulocytes
Granulocytes are a subgroup of white blood cells distinguished by their characteristic granules – small, grain-like structures in the cytoplasm. These granules contain various substances that are released during immune defense and other processes. The granulocytes include neutrophils, eosinophils, and basophils, each of which plays distinct roles in the immune system, particularly in fighting infections and managing allergic reactions. In this description, we will focus on neutrophils, as they make up 50-70% of leukocytes in human blood, making them the most abundant immune cells.
Neutrophils, also known as neutrophil granulocytes, continuously circulate in the bloodstream and are among the first immune cells to respond to an infection. When pathogens invade the body, chemotactic signals are released, attracting neutrophils to the site of infection. In response, they exit the bloodstream and migrate into the tissue, where they can reach the infection site within minutes.

On-site, neutrophils identify pathogens using their pattern recognition receptors (PRRs), which detect specific pathogen-associated molecular patterns (PAMPs) present on invaders like bacteria, viruses, and fungi. Their PRRs also recognize damage-associated molecular patterns (DAMPs), which are released by damaged or stressed cells. This dual recognition allows neutrophils to respond not only to invading pathogens but also to cellular damage within the body.

Pattern recognition receptors (PRRs)
Pattern recognition receptors (PRRs) are a key component of the innate immune system. They are responsible for the rapid detection of pathogens and cell damage, triggering an immediate immune response. PRRs recognize specific molecular patterns associated either with pathogens or with cell damage. This recognition allows the immune system to quickly identify and respond to potential threats.
These molecular patterns are divided into two main categories:
Pathogen-associated molecular patterns (PAMPs): These molecules are typical for microorganisms such as bacteria, viruses, fungi and parasites, but not for human cells.
Damage-associated molecular patterns (DAMPs): These molecules are released by stressed or damaged cells and signal a danger to the organism.
PRRs can be divided into different classes, each of which specialises in different types of PAMPs and DAMPs:
Toll-like receptors (TLRs)
TLRs are specialized sensors located on the surface of immune cells such as macrophages, dendritic cells and neutrophils, as well as within their internal compartments. TLRs recognise a wide range of PAMPs:
TLR1 and TLR2: These two often work together and recognise certain molecules on the surface of bacteria.
TLR3: This receptor recognises viral RNA, i.e. the genetic material of viruses.
TLR4: It recognises lipopolysaccharides, which occur on the surface of many bacteria.
TLR5: This receptor recognises flagellin, a protein found in the flagella of bacteria.
TLR6: Often works together with TLR2 and also recognises molecules on bacteria.
TLR7 and TLR8: Both recognise viral RNA, similar to TLR3.
TLR9: It recognises DNA from bacteria and viruses that exhibit certain patterns.
NOD-like receptors (NLRs)
NLRs are intracellular receptors found in the cytoplasm of cells. They act like tiny sensors, constantly scanning for signs of infection or cellular damage. These receptors detect specific molecules either originating from pathogens, such as bacteria, or from within our own cells when they are stressed or damaged.
RIG-I-like receptors (RLRs)
RLRs are also intracellular receptors that are localised in the cytoplasm of cells. They are specialised in recognising viruses that have entered the cell.
C-type lectin receptors (CLRs)
CLRs are located on the cell surface of immune cells such as macrophages and dendritic cells. They recognise certain sugar structures (carbohydrates) on the surface of pathogens, particularly fungi.
Neutrophil granulocytes eliminate intruders or diseased cells by engulfing and destroying them. They encapsulate the pathogen in a membrane-bound vesicle called a phagosome. After ingestion, the granules of the neutrophils fuse with the phagosome. These granules contain enzymes and antimicrobial substances that degrade and kill the enclosed pathogen. The remaining breakdown products that cannot be reused are then released from the neutrophils. These remnants can be taken up and further processed by other immune cells, such as macrophages.
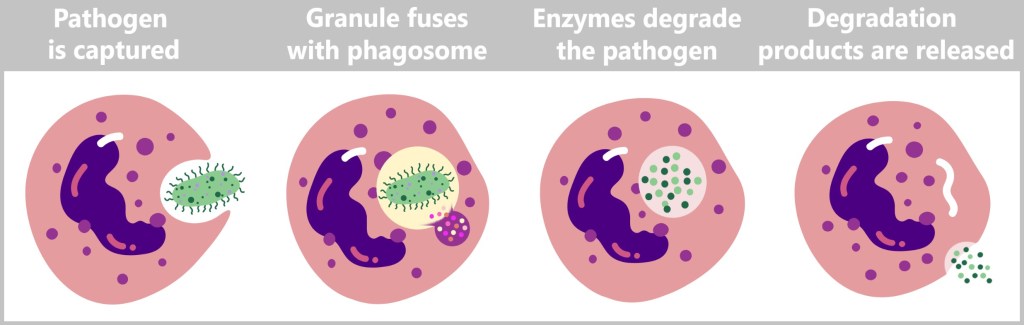
In addition, neutrophils also eliminate damaged tissue cells. This entire process, in which cells engulf and degrade foreign bodies and damaged cells, is referred to as phagocytosis.
The term phagocytosis originates from Greek and consists of two parts: phagein, meaning „to eat” or „to engulf”, and kytos, meaning „cell” or „container”. Thus, the term „phagocytosis” literally describes „cell-eating” or „engulfing by cells”. For this reason, cells that perform this function are also referred to as phagocytes.
Neutrophils have a relatively short lifespan and often die after phagocytosis and digestion of pathogens. This process, called apoptosis (programmed cell death), helps to regulate the inflammatory response.
When many neutrophils die in an infected area, their remains accumulate along with the digested pathogens and cellular debris. This accumulation of dead cells and degradation products forms pus. Pus is a viscous, yellowish or greenish fluid that is often found in infected wounds or abscesses. Apoptotic (dead) neutrophils are also absorbed and digested by macrophages, cleansing the surrounding tissue and promoting healing.
In some cases, neutrophils release net-like structures consisting of DNA and antimicrobial proteins. These NETs (Neutrophil Extracellular Traps) trap pathogens and kill them by preventing their movement and replication.

This short animated video shows very nicely how neutrophils work.
Neutrophils work closely with other immune cells such as macrophages and dendritic cells by releasing signalling molecules. These signalling molecules, such as chemokines and cytokines, recruit and activate other immune cells to the site of infection. Neutrophils thus make a significant contribution to the inflammatory response. Their rapid response and ability to effectively combat pathogens makes them a crucial component of the innate immune response.

5.3. b) Macrophages
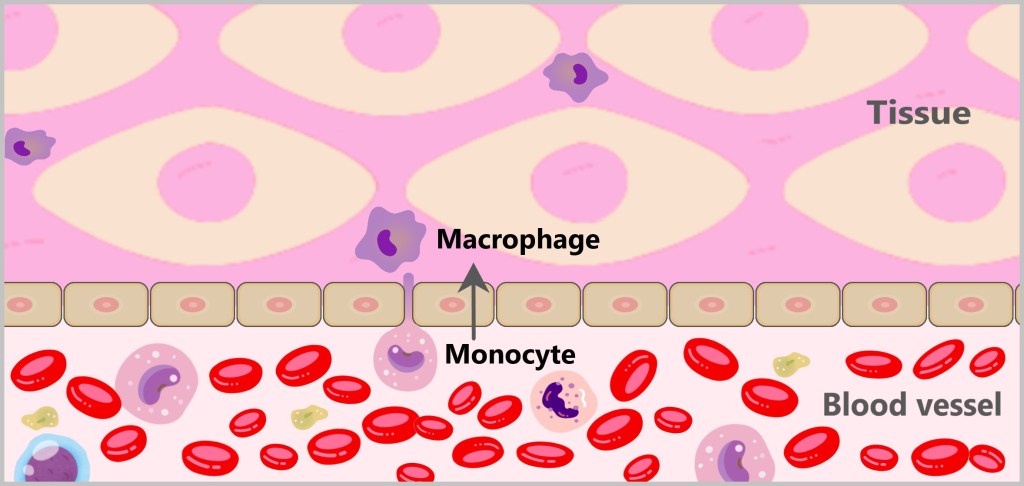
Macrophages are large phagocytic cells, with their name derived from the Greek word ‚macro‘ meaning large and ‚phage‘ meaning eater. There are different types of macrophages.
Resident macrophages are firmly anchored in the tissues. Recruited macrophages arise from the monocytes circulating in the blood. When monocytes come into contact with cytokines (signaling molecules), they migrate from the blood into the tissue and differentiate into macrophages there. Once in the tissue, they often remain stationary while patrolling for pathogens and dead cells.
Macrophages recognize pathogens through specific proteins on their surface called pathogen-associated molecular patterns (PAMPs). These surface proteins of pathogens fit into the pattern recognition receptors (PRRs) of macrophages like a key fits into a lock. Similarly, cell debris or dead cells produce specific „eat-me” signals, which are recognized by macrophages in the same way. Once the pathogen or cell debris is identified, the macrophage binds to it, envelops it with its flexible membrane, and initiates phagocytosis.
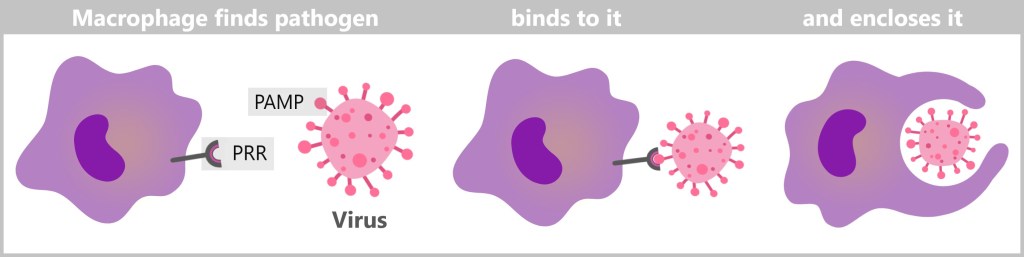
During phagocytosis, the macrophage absorbs the pathogen or cell debris by invagination and forms a membrane-enveloped vesicle, the phagosome. This phagosome fuses with a lysosome to form a phagolysome. In the phagolysome, the ingested particles are broken down by the digestive enzymes of the lysosome. Here you can see macrophages in action.

Phagocytosis is an ancient mechanism dating back to unicellular eukaryotes such as amoebae and provides a vivid example of how the immune system has evolved over millions of years.
Phagocytosis: an ancient survival mechanism
Phagocytosis is a good example of how the immune system has developed over millions of years.
The history of phagocytosis goes back a long way and is closely linked to the evolution of life. Single-celled organisms such as amoebae have been using phagocytosis to ingest food for more than 100 million years. To detect their food, they have receptors that react to specific chemical substances released by bacteria or other potential food sources. Amoebae move towards these signals through a process called chemotaxis. They then enclose their prey with their cell membrane and form so-called food vacuoles in which digestion takes place.
Amoebae are therefore excellent examples of the early users of phagocytosis. They are ancient life forms that are simple in their structure but very effective in their function. The phagocytosis mechanism may even have served evolutionarily as the basis for the cell fusion process that eventually led to the emergence of the eukaryotic cell, which made more complex organisms possible.
Sources:
The Origins of Phagocytosis and Eukaryogenesis
The Endosymbiotic Theory
Discovery by Ilya Metschnikow
The actual term ‘phagocytosis’ was first coined at the end of the 19th century by the Russian biologist Ilya Metschnikow, who is regarded as a pioneer of immunobiology. During a stay in Italy, Metschnikow carried out studies on starfish and observed how certain cells devoured pathogens or foreign particles. He called these cells ‘phagocytes’ (Greek: ‘scavenger cells’), and the process itself he termed ‘phagocytosis’. For this discovery, Metschnikow was awarded the Nobel Prize for Medicine in 1908.
Evolution of phagocytosis in the immune system
Over the course of evolution, phagocytosis has evolved from a mechanism for nutrient uptake into a central component of the immune system. Immune cells such as macrophages, dendritic cells, and neutrophils in the innate immune system have refined the process of phagocytosis, using it not only to recognize, engulf, and destroy pathogens but also to communicate with other immune cells.
The „parading” of antigens on the cell surface is a true evolutionary trick: immune cells present fragments of digested pathogens (antigens) to alert other immune cells. While amoebas simply digest their prey, immune cells have learned to turn this into a „message” for the body!
Macrophages mainly concentrate on „eating” pathogens and „cleaning up” cell debris. However, this also gives rise to the possibility of antigen presentation.
During degradation, smaller fragments are released, including potential antigens, which are bound to MHC class II molecules and presented on the cell surface. This antigen presentation is crucial for the activation of other immune cells, especially T cells. The antigen-MHC II complexes are presented to the T cells, which then initiate the specific immune response – a process that we will discuss in more detail in the following sections.

Many other smaller molecules produced by the digestive process, such as amino acids, sugars and lipids, can be re-utilised by the macrophage as nutrients.
Other waste products and indigestible residues are packed into vesicles and excreted from the cell. This prevents the accumulation of waste within the macrophage and allows it to continue working. Some of the indigestible residues can ultimately be removed via various excretory processes of the body, such as urine, faeces or sweating. This occurs after further degradation and transport through the lymphatic system or the bloodstream to the corresponding organs such as the kidneys or intestines.
Macrophages are very efficient phagocytes and can ingest and degrade a large number of pathogens and cellular waste. Within a few hours, one macrophage can phagocytise hundreds of bacteria.
As not just one pathogen usually invades the body, macrophages call on other immune cells for help. To do this, they secrete cytokines that strengthen the immune response.
Cytokines cause the blood vessels around the site of infection to dilate, leading to increased blood flow. This enhanced circulation allows more immune cells, oxygen, and nutrients to reach the affected area. As a result, swelling (edema) occurs in the tissue, which also explains the redness and warmth often associated with inflammation.
As the immune response progresses, macrophages initiate the healing process by releasing growth factors that promote cell division and differentiation.
Macrophages play a central role in the immediate immune defense. Their primary tasks are fighting and eliminating pathogens, as well as clearing cellular debris through phagocytosis. To accomplish this, macrophages possess around 60 different types of receptors, enabling them to recognize a wide variety of pathogens. Additionally, they perform antigen presentation to support the specific immune response, though this is more of a supplementary function. In contrast, dendritic cells are more specialized in collecting and presenting antigens, with their primary role being the activation of the adaptive immune response.

5.3. c) Dendritic Cells
Dendritic cells are specialised immune cells that play a key role in triggering the specific immune response. The name is derived from their numerous, branched projections, which are reminiscent of the dendrites of nerve cells.
Dendritic cells originate from precursor cells in the bone marrow and further differentiate in tissues. They are found in almost all tissues of the body, especially at the interfaces with the external environment, such as the skin, respiratory tract, and gastrointestinal tract, where they continuously search for antigens. In tissues, they can develop into different subtypes, each of which has specific roles in recognizing and presenting antigens.
Dendritic cells recognise pathogens and foreign substances through special receptors on their surface, the so-called pattern recognition receptors (PRRs). These receptors bind to characteristic molecular patterns on the pathogens, which are known as pathogen-associated molecular patterns (PAMPs). Damaged or dead cells also send out specific „eat-me” signals that are recognised by the dendritic cells.
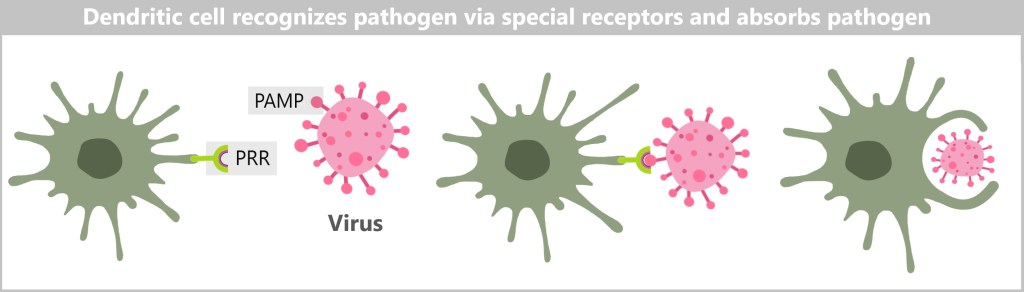
As soon as dendritic cells have taken up pathogens or cell debris, they undergo a maturation process. This process begins with the PRRs recognising the PAMPs on the pathogens. After this recognition, the dendritic cells phagocytose the pathogens or cell debris and enclose them in a membrane-enveloped vesicle, the phagosome. Within the phagosome, the ingested materials are broken down by digestive enzymes.
During this degradation, smaller fragments called antigens are produced. These antigens are then bound to MHC class II molecules and presented on the cell surface.

Following the uptake and processing of antigens, dendritic cells migrate to the nearest lymph nodes, where they present the antigens to the T cells and thus initiate the activation of the specific immune response.
Although dendritic cells also play an important role as phagocytes in the innate immune defence, their main function as antigen-presenting cells (APCs) is to activate the specific immune defence. We will therefore deal with their role as APCs in detail in the chapter ‚The specific immune defence‘.
In addition to activating the specific immune response, dendritic cells can also have a regulatory effect. Depending on the context and the signals received, they can take on both immunostimulatory and immunosuppressive functions and thus contribute to the fine-tuning of immune responses.

5.3. d) Natural Killer Cells
Natural killer cells (NK cells) are a type of lymphocyte that belongs to the leukocytes (white blood cells). They develop from lymphoid stem cells in the bone marrow and are an essential component of the innate immune response.
NK cells circulate in the blood and are tasked with inducing apoptosis (programmed cell death) in specific cells, particularly virus-infected cells and tumor cells. Their presence in the bloodstream allows them to rapidly respond to infections and migrate into different tissues when a threat arises. NK cells are also found in various tissues and organs throughout the body, particularly in the spleen, liver, lymph nodes, and lungs.
Although NK cells do not need to be activated, their activity is enhanced by cytokines. Unlike other immune cells, they do not possess specific receptors for foreign antigens. Instead, they use a variety of receptors, which are divided into two categories: activating and inhibitory receptors.
Inhibitory receptors recognize MHC class I molecules, which are normally present on the surface of all nucleated cells and serve as SELF-markers for the immune system. The binding of inhibitory receptors occurs independently of the peptides presented on the MHC class I molecule; the NK cell does not directly recognize the peptides but rather the MHC class I molecule itself.
NK cells scan cell surfaces in the body and bind their inhibitory receptors to MHC class I molecules. This binding sends an inhibitory (negative) signal into the NK cell, which suppresses its activation and thus protects healthy cells from attack.
Tumor cells and virus-infected cells often reduce the expression of MHC class I molecules to evade detection by cytotoxic T cells. This phenomenon is known as the „missing-self” principle. When a cell expresses no or reduced MHC class I molecules on its surface, the inhibitory (negative) signal is not transmitted, allowing the NK cell to recognize the target cell as abnormal and attack it.

Activating receptors recognize stress molecules expressed on the surface of infected or transformed cells. There are various stress molecules collectively referred to as„stress-induced self”.When cells are under stress, they express these molecules. The activating receptors on NK cells recognize these stress molecules, leading to the activation and attack of NK cells on the affected cells.
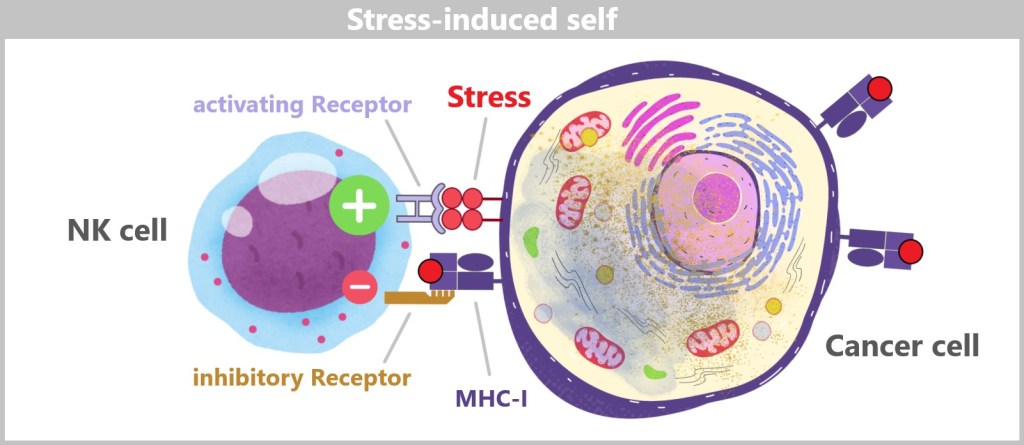
The activation of NK cells depends on a delicate balance between activating and inhibitory signals. When both activating and inhibitory signals are detected, a weighting of these signals occurs. In the case of predominant inhibition from the inhibitory receptors, the activation of the NK cell is suppressed, and the target cell remains protected. However, if the activating signals dominate or the inhibitory signals are weaker, the NK cell is activated and attacks the target cell. In this way, NK cells can recognize and kill their targets despite the efforts of target cells to evade immune surveillance.
The natural killer cell (NK cell) can eliminate the target cell in two ways.
1) Killing by perforins and granzymes
Firstly, it releases cytotoxins. The cytoplasm of the NK cell contains numerous small granules that contain proteins such as perforin and granzymes.

These are released in the vicinity of the target cell. Perforins create pores in the membrane of the target cell, through which granzymes enter and initiate apoptosis.

Sometimes antibodies (produced by the immune system) bind to abnormal cells, such as virus-infected cells or cancer cells. NK cells do not recognize the specific variable part of the antibody that binds to the antigen but instead recognize the constant tail region of the antibody, known as the Fc region. At this point, it is enough to know that antibodies consist of a variable part and a constant part. We will examine antibodies in more detail in later chapters. NK cells have Fc receptors that specifically bind to this constant Fc region of antibodies.
After the NK cell has bound to the antibodies, it releases perforins and granzymes to destroy the target cell.

2) Killing by Fas Ligand (FasL)
Tumor cells or infected cells can express Fas receptors. Fas is a specific „death receptor”. NK cells possess Fas ligands (FasL). When the NK cell binds its Fas ligand to the Fas receptor on the target cell, it transmits signals that activate the apoptotic pathway in the target cell. The interaction between Fas ligand and Fas receptor is also referred to as the „kiss of death”.

The target cell undergoes a series of ordered changes: First, it shrinks and the chromatin (the material that makes up the chromosomes) becomes denser. Then the DNA is broken down into small fragments. Finally, the cell disintegrates into small vesicles, so-called apoptotic bodies, which are recognised and eliminated by specialised cells of the immune system, such as macrophages.

The following animated video briefly and concisely explains the function and importance of NK cells.
„In the recent past, it was assumed that NK cells do not have an immunological memory, i.e. they cannot ‚remember‘ encounters with virus-infected cells. For some years now, however, there have been indications that they adapt to their environment over the course of their lives – abilities that were previously only attributed to the acquired immune system.“ [DocCheck]
Although NK cells are part of the innate immune system, they are often regarded as a bridge between the innate and adaptive immune systems, as they influence the overall immune response through interaction with other immune cells. Natural killer cells play a crucial role in combating viral infections and cancer cells, keeping these threats in check until the specific immune response is fully activated.
The leukocytes (white blood cells) as the second line of defence are supported in their work by the complement system.
5.4. The Complement System
An important part of our immune response is the complement system. It is present in newborns and thus belongs to the innate immune system. As an immediate defense, it targets invaders that have breached the body’s natural barriers, such as the skin or mucous membranes. In cooperation with the specific immune system, it „complements” the immune response.
Essentially, the complement system has three tasks: it marks enemies, activates the immune defence and burns holes in the enemies until they die.
In order to get rid of pathogens, the complement system goes through the following phases:
a) Recognition and marking of the pathogen (opsonization)
b) Activation of the immune defence (inflammation, complement cascade)
c) Destruction of the pathogen (phagocytosis, lysis)
d) Removal of the debris and healing of the damaged tissue
For these tasks, around 30 complement proteins are available, which are present in body fluids and tissues. These proteins have different functions and are called complement factors (C1, C2, C3, … C9). They are capable of splitting into further subunits (e.g., C3 becomes C3a and C3b). They are activated by the cleavage.
a) Recognition and marking of the pathogen
Pathogens are recognized by unique surface patterns that are only found on foreign microorganisms or diseased cells. Complement proteins continuously patrol the blood, tissues, and mucous membranes, scanning for pathogenic changes. As soon as they encounter something foreign that they identify as pathogenic, they attach to the identified target and label it for the immune system as a pathogen.

For example, the finding may be an immune complex (IC), in which an antibody has bound to an antigen. These complexes can also attach to the surfaces of bacteria, viruses, and other parasites, a process known as opsonization. Pathogens labeled in this way become more visible and accessible to other immune cells, such as macrophages, dendritic cells, granulocytes, and natural killer cells. Depending on the pathway of opsonization, there are three different complement pathways.

b) Activation of the immune defence
Depending on the activation pathway, different proteins bind to the pathogen (see figure above). In the classical pathway, C1 binds to immun complexes (IC), in the lectin pathway, mannose-binding lectin (MBL) binds to sugar structures (mannose) on the pathogen surface, and in the alternative pathway, C3 binds directly to the pathogen surface. These bindings all lead to the formation of C3 convertase, a special enzyme that splits the protein C3 into two parts: C3a and C3b.
Since this mechanism is complex, it is only briefly mentioned here that in the classical and lectin pathways, a series of reactions occurs after the binding of the corresponding proteins, ultimately leading to the formation of C3 convertase. In the alternative pathway, C3 convertase forms directly. C3b, the product of this cleavage, then attaches to the surface of the pathogen. In the alternative pathway, C3b remains directly on the pathogen surface.
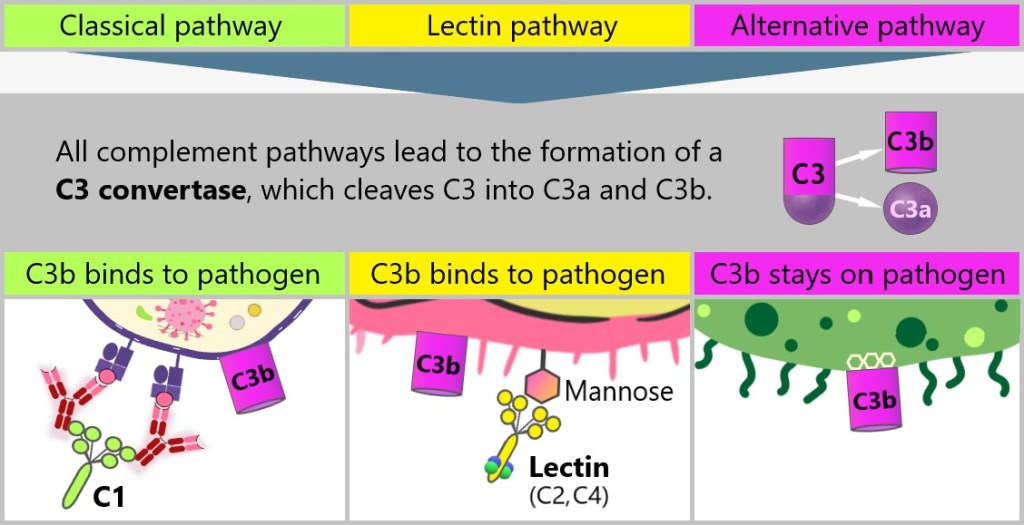
The smaller C3a proteins act as distress signals, which are flushed away and activate additional complement proteins. These newly mobilized proteins follow the distress signals to the source of the inflammation, attach to the enemy, and also undergo cleavage. This leads to a domino effect known as the complement cascade. An inflammatory response is triggered, which continues to amplify until countless complement proteins cover the invaders.
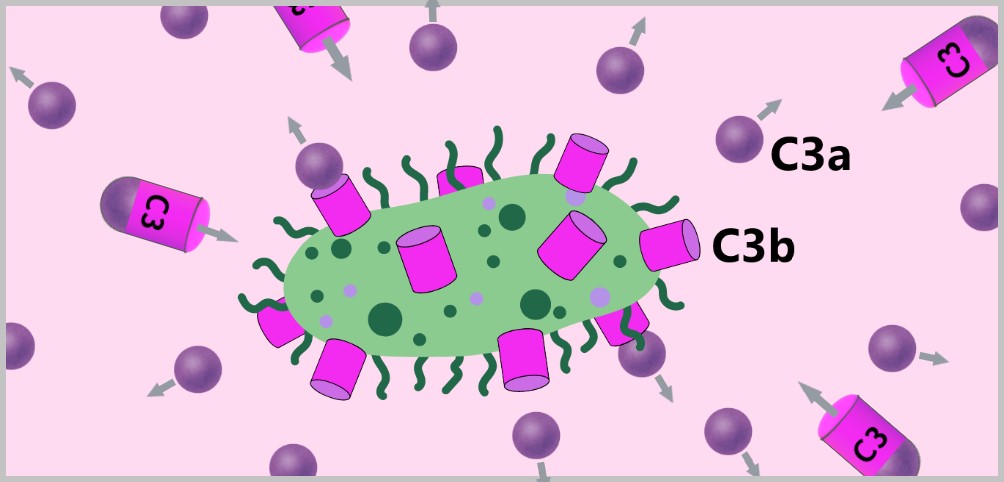
The distress signals also cause the smaller local blood vessels to dilate and become more permeable. This makes it easier for immune cells to migrate from the blood vessels into the infected tissue. In addition, further immune cells are attracted by the distress signals. This leads to swelling of the surrounding area at the site of infection, resulting in the typical characteristics of inflammation such as swelling, redness, warmth and pain. The inflammation burns.

c) Destruction of the pathogen
The summoned immune cells locate the marked pathogens by utilizing specific complement receptors on their surface that bind to the C3b protein. These receptors enable phagocytes to selectively recognize the marked pathogens. The phagocytes then engulf the pathogen, take it in, and digest it.

The complement proteins also launch an attack. Additional proteins (C5b, C6, C7, C8, and several C9) now bind to the invader. These complement proteins assemble into protein units (C5b678poly9 complexes), known as membrane attack complexes (MAC), which punch pores in the enemy’s cell membrane. The holes created in the cell membrane lead to a fluid exchange between the interior of the cell and the surrounding environment, resulting in the dissolution of the pathogen, a process known as lysis.

The animation „Little Bombs in the Blood – The Complement System” vividly illustrates the complex processes described.
The complement system interacts strongly with the coagulation system. The inflammatory response triggered by the complement system causes blood clotting at the site of infection. Clots block the small blood vessels, preventing the pathogens from spreading further through the blood. At the same time, fluid is directed into the tissue and from there to the lymph nodes. This allows the dendritic cells loaded with antigens to more easily reach the lymph nodes. There, they present the antigens to the T cells, thereby initiating the specific immune response.
d) Removal of the debris and healing of the damaged tissue
The immune response ends when the threat has been eliminated. In order to heal, the remains of cell debris, bacteria or viruses must first be removed from the wound area. This task is performed by the macrophages. The inflammation dissolves as a result of the cleansing. With the formation of new cells, the tissue is repaired or completely regenerated. The clots that have become superfluous are broken down again by fibrinolysis, i.e. the enzymatic dissolution of clots.
Regulation of the complement system
The various complement fragments also have an immunoregulatory effect. These regulators can enhance or inhibit the defense response, thereby terminating the immune response. A dysregulation of the complement system has serious consequences for health. Both the complement and coagulation systems are meant to act only locally. Widespread activation throughout the entire organism could have severe consequences. Dysregulation can lead to autoimmunity, where the body’s own cells are attacked, resulting in various diseases.
The combination of leukocytes and the complement system ensures an effective and coordinated defense against infections. If the specialized immune cells, in collaboration with the complement system, manage to eliminate all pathogens, the immune response comes to an end. However, it is not always possible to completely eradicate all pathogens through the nonspecific defense. In such cases, the specific immune system comes into action.
5.5. The Specific Immune Defense
Although the innate, non-specific defence reacts very quickly, it is not always sufficient. It can fend off many pathogens, but some are able to overcome this line of defence. From this point onwards, the body reacts with a targeted, i.e. specific, immune response. The specific defence is slower than the non-specific defence, as it needs time to develop a tailored response to the invader.
As this defence is specifically directed against certain pathogens, it is much more accurate and efficient. It can also memorise information about an attacker. It develops in a lifelong training process. This is why it is also referred to as acquired immune defence or adaptive immune defence.
5.5.1. Key Players of the Adaptive Immune Response
5.5.2. Naive B and T Cells: The Diversity of the Immune Response
5.5.3. The Role of Antigen-Presenting Cells (APCs)
5.5.4. The Importance of Lymph Nodes for the Adaptive Immune Response
5.5.5. Recognition Phase
5.5.6. Activation Phase
5.5.7. Effector Phase
III – Third Line of Defense: The Antibodies
5.5.8. Types of Antibodies
5.5.9. The Action Phase of Antibodies
5.5.10. Regulatory T Cells and Their Role in the Immune System
5.5.11. Switch-Off Phase
5.5.12. Immunological Memory
Before we go into the individual processes in more detail, the following section will introduce the individual players in the specific immune defence.
5.5.1. Key Players of the Adaptive Immune Response
Key players in the adaptive immune response are specialized leukocytes known as B lymphocytes (B cells) and T lymphocytes (T cells). These work within two closely intertwined systems: the humoral and the cellular immune response.

a) Development and Maturation of Lymphocytes
b) Humoral and Cellular Defense Mechanisms
c) Migration and Distribution of Lymphocytes
d) Structure of the Lymphatic Organs
a) Development and Maturation of Lymphocytes
B and T cells develop from stem cells in the bone marrow. While the B cells fully mature in the bone marrow, the T cells migrate to the thymus after their formation in order to complete their maturation there. The abbreviations B and T are derived from their places of formation: B stands for Bone marrow and T for Thymus.
B and T cells are considered naive as long as they have not yet encountered an antigen.
b) Humoral and Cellular Defense Mechanisms
Humoral defence involves combating pathogens in body fluids such as blood and lymph (from the Latin humor, meaning fluid). The main players in this defense are B lymphocytes. They recognize specific antigens and subsequently produce antibodies that bind to the antigens. This binding marks the pathogens for destruction by other immune cells or directly neutralizes them. This process is particularly effective against pathogens that are outside of body cells, such as bacteria and viruses that have not yet invaded a cell.
Cellular defence, on the other hand, is carried out by T lymphocytes. They recognise and destroy infected cells or cells that have become abnormal in some other way. T lymphocytes can also activate other immune cells to enhance the destruction of pathogens. T cells are further subdivided into CD4+ T cells and CD8+ T cells, each of which has different roles in the immune response.
In the following text, the terms CD4 T cells and CD8 T cells will be used to improve readability. The ‚+‘ symbol denotes the expression of specific surface markers on the T cells. It indicates that these T cells carry the corresponding markers on their surface. In many contexts, the ‚+‘ symbol can be omitted without losing its meaning.
CD4 T cells are primarily T helper cells; they support and regulate the activities of other immune cells. CD8 T cells are mainly cytotoxic T cells. They recognize and destroy infected cells, especially those infected by viruses, as well as cancer cells.
c) Migration and Distribution of Lymphocytes
After their development, the naive B cells and T cells enter the blood. As soon as they reach a secondary lymphatic organ (e.g. a lymph node), they leave the blood and migrate through the lymphatic tissue. From there, they return to the blood via the lymphatic vessels and thus shuttle back and forth between the blood and secondary lymphatic tissue.
To understand this better, it is worth taking a closer look at the structure of the lymphatic organs.
d) Structure of the Lymphatic Organs
The various secondary lymphatic organs (such as lymph nodes, spleen, etc.) are all organised according to a similar pattern: they contain separate areas in which the B and T cells accumulate – the so-called B cell and T cell zones. Circulating T and B cells enter the secondary lymphoid tissues from the blood via a common pathway, but are directed to their respective compartments (areas) by the action of different chemokines (signalling proteins that control the movement of cells).

A lymph node is surrounded by a thin capsule of connective tissue that extends small partitions into the interior, dividing the lymph node into different sections. On the convex side, lymph fluid flows into the lymph node through afferent lymphatic vessels. On the indented side, known as the hilum, the filtered lymph fluid exits the lymph node through a special efferent lymphatic vessel. At this point, blood vessels also enter and exit the lymph node. Inside, the lymph fluid passes through specialized channels known as the sinus system. In the outer layer of the lymph node, B cells are found in small clusters called lymph follicles. This B cell region directly borders the area of T cells, known as the paracortex. Both B and T cells can enter the lymph node through the blood (artery) or the afferent lymphatic vessels and then exit through the blood (vein) or the efferent lymphatic vessels.
The lymphatic system
The lymphatic system is an essential component of our immune system and consists of a network of lymphatic vessels and lymph nodes that permeate the entire body. Unlike the blood vessel system, it is not closed. Lymph, a clear, slightly yellowish fluid, is made up of water, proteins, fats, and white blood cells.
Body cells require nutrients and oxygen to perform their various functions, while simultaneously needing to dispose of metabolic waste. Arteries supply fresh blood, while veins transport used blood and harmful substances away. Lymph (from the Latin for ‚clear water‘) serves as an intermediary between blood and body cells. It exits from the capillaries, the thinnest blood vessels, into the space between the cells and flows freely there, taking up metabolic products, fats, cell debris, and pathogens.
The lymph first collects in fine vessels and then flows into larger lymphatic channels. Finally, it flows into the two main venous branches at the base of the neck. On its way there, the lymph passes numerous lymph nodes, which are distributed along the lymph vessels. The lymph nodes act like filter stations that cleanse the lymph by trapping pathogens.
Lymph nodes are small, bean-shaped structures that typically range from a few millimeters to about 1 cm in size. A healthy adult has approximately 600 to 700 lymph nodes distributed throughout the body. They not only filter excess fluid from the tissue but also activate the immune system as soon as pathogens are detected, initiating the defense response to keep the body healthy.
The primary lymphoid organs are the bone marrow and the thymus. Lymphocytes (B cells and T cells) are formed from stem cells in these organs.
The secondary lymphatic organs include the lymph nodes, lymphatic vessels, spleen, and lymph follicles of the mucous membranes. These organs act as filtration stations, removing dead cells, foreign material, and protein aggregates from the circulation. In these organs, lymphocytes, monocytes, and dendritic cells are highly mobile and can move in and out of the lymphatic tissue.
5.5.2. Naive B and T Cells: The Diversity of the Immune Response
Naive T cells carry unique T cell receptors (TCRs) on their surface that are specifically designed for the recognition of antigens. Similarly, naive B cells possess a variety of B cell receptors (BCRs), which also serve the purpose of antigen recognition.
The immense diversity of TCRs and BCRs arises from a genetic mechanism known as somatic recombination or somatic V(D)J recombination. In this process, different gene segments are randomly combined, leading to the formation of billions of different receptors. This enables the immune system to be prepared for nearly every conceivable antigen.
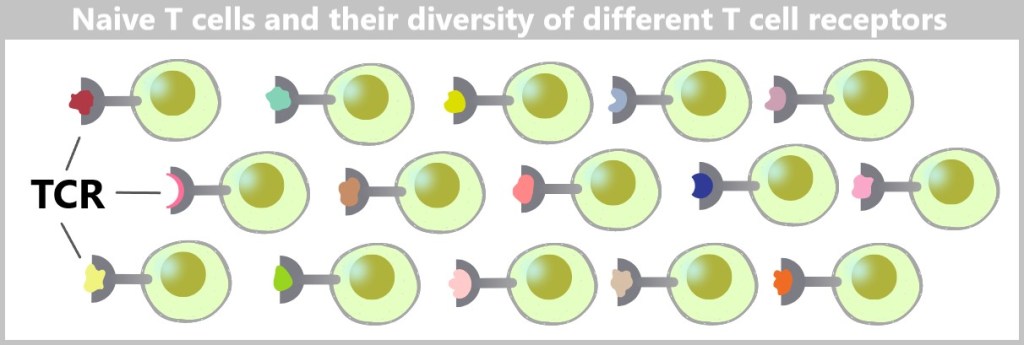
What is somatic V(D)J recombination?
Our immune system must be capable of recognizing millions of different pathogens, such as viruses and bacteria. For this to be possible, we need a vast number of different „recognition tools”: antibodies and T cell receptors. These tools are specialized proteins that must fit precisely to a specific pathogen, much like a key fits into a lock. But our genetic material (DNA) is limited – so how can the body produce so many different recognition tools?
The solution lies in a clever mechanism called somatic V(D)J recombination. This mechanism works like a construction set:
Genes as a Blueprint: Antibodies and T cell receptors are made up of different building blocks that are encoded by specific gene segments. These segments are called V (Variable), D (Diversity), and J (Joining). Each of these segments specifies a part of the antibody or T cell receptor.
Mix and Match: Somatic V(D)J recombination occurs in the precursor cells of B and T cells. In this process, individual V, D, and J segments are randomly combined to create a unique blueprint for an antibody or T cell receptor. This results in millions of different combinations – each combination corresponds to a unique antibody or receptor.
Random Diversity: The process of recombination is designed to create a significant amount of diversity. Even tiny changes in the combined segments result in a different antibody or receptor. This allows the immune system to be prepared for virtually every conceivable pathogen.
Why is this important?
Somatic V(D)J recombination allows the immune system to produce an incredibly wide range of antibodies and T-cell receptors without each of these ‚recognition tools‘ having to be stored in our DNA. This means that our immune system is flexible and adaptable and can also respond to new or unknown pathogens.
Summary
Somatic V(D)J recombination is like a huge construction set that enables the immune system to create millions of different „keys” (antibodies and T cell receptors). Each key is unique and can recognize and combat a specific pathogen. This allows our body to effectively respond to a variety of threats, even if we have never encountered these pathogens before.
You can find detailed information on this complex topic in the following sources:
„How-to make: a T cell receptor (the simpler version)“
„TCR gene rearrangements“
From birth, humans possess at least one immune cell for every potential pathogen on this planet. [Evolution of immune receptor diversity]
Or as it is impressively described in the videos „How The Immune System ACTUALLY Works” and „You Are Immune Against Every Disease”: „Your immune system has a perfect weapon against every possible disease in the universe against the Black Death the Corona virus or an infection that will emerge in 100 years on Mars.”
The videos were published on the channel ‚Kurzgesagt – In a Nutshell‘ in 2021, which was part of the funk network from ARD and ZDF until the end of 2022. [wikipedia]
This diversity gives the adaptive immune system its impressive strength by maintaining a vast library of responses to virtually every possible pathogen. This immense adaptability is crucial for being able to combat rapidly evolving bacteria and viruses.
Despite its remarkable diversity, our immune system also reaches its limits.
A vivid example of this is the plague in the Middle Ages. The bacterium Yersinia pestis, which caused the disease, presented a novel and deadly threat. The infection spread so quickly that the immune system of those affected often couldn’t mount an effective defense in time before the disease proved fatal.
Individual differences in the immune response are based on a variety of genetic factors. Not everyone has the same ability to respond to infections, which explains why some people are better able to fight off pathogens than others. An important factor here is the diversity of MHC molecules that control the recognition and presentation of pathogens. People with different MHC variants can react differently to infections. While some are extremely susceptible to certain pathogens, there are always people who are better able to deal with new threats thanks to their genetic make-up. This genetic diversity was one of the reasons why not all people died during the plague epidemic – some had naturally stronger defence mechanisms.
In addition, factors such as age, pre-existing conditions and general health influence the performance of the immune system. If the immune system is weakened or overloaded, even with all its complexity and adaptability, it may not be able to react quickly enough to stop a deadly infection.
Ultimately, the immune system is an impressive defense mechanism that protects us against countless threats. However, there are limits, reminding us that genetics, environmental factors, and the principle of chance can determine who survives an infection – and who does not.
All living organisms consist mainly of proteins, which are structured like 3D puzzle pieces and can take on billions of different shapes. Pathogens utilise this diversity to constantly change their structures. The influenza virus, for example, mutates so quickly that its envelope proteins are constantly changing slightly, making it difficult for the immune system to recognise it.
The innate immune system recognizes many of the most common protein puzzle pieces and thus acts as a general-purpose weapon. However, it is often powerless against the countless mutations and adaptations of pathogens. This is where the adaptive immune system comes into play, as it can distinguish between one and ten billion different hostile protein puzzle pieces.
However, in order for these highly specialised cells to effectively combat a specific pathogen, they must first be presented with the corresponding antigen. This is precisely where the antigen-presenting cells (APCs) play a decisive role.
5.5.3. The Role of Antigen-Presenting Cells (APCs)
Antigen-presenting cells (APCs) form the crucial interface between the innate and adaptive immune responses. Among the most well-known APCs are macrophages, dendritic cells, and B cells, with dendritic cells being particularly efficient at capturing and presenting antigens. Antigen presentation is a fundamental requirement for initiating the adaptive immune response, especially for activating T cells, which usually occurs in the lymph nodes. In the lymph nodes, antigen-presenting cells and T cells come into contact, enabling the immune response to take place.
Dendritic cells have the unique ability to present antigens through two distinct pathways: via MHC class II molecules and MHC class I molecules. Let’s take a closer look at both presentation pathways.
a) Antigen Presentation via MHC-II Molecules
b) Antigen Presentation via MHC-I Molecules
c) Dendritic Cells migrate to the Lymph Nodes
a) Antigen Presentation via MHC-II Molecules
As soon as a dendritic cell has recognised an (exogenous) pathogen, it binds to it, encloses it with its mobile membrane and absorbs the pathogen by invaginating it.
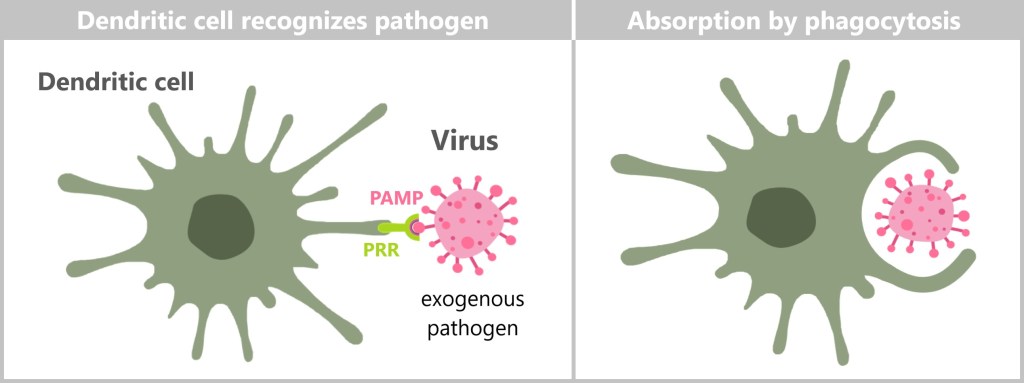
The pathogen ends up in a membrane-enveloped vesicle known as a phagosome. The phagosome fuses with a lysosome, which contains digestive enzymes that break down the pathogen. Smaller fragments are produced in the phagolysosome, including potential antigens. These antigens are bound to MHC class II molecules and presented on the cell surface.

1) Phagocytosis of exogenous antigens. 2) Degradation of antigens in phagolysosomes. 3) Assembly and transport of MHC-II molecules from the endoplasmic reticulum (ER) to the Golgi apparatus. 4) Fusion of the vesicles and loading of the MHC-II molecules with peptides. 5) Presentation of the loaded MHC-II on the cell surface.
In this way, the dendritic cell displays outward what pathogen it has detected. These MHC-II-antigen complexes are crucial for the activation of CD4 T cells.
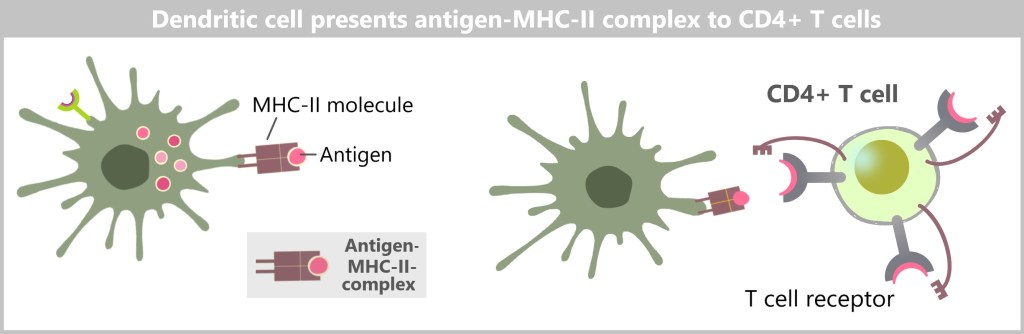
A clear and concise visualization of MHC class II antigen processing and presentation is provided by Dr. Noel Kowal.
b) Antigen Presentation via MHC-I Molecules
I – Direct infection
Dendritic cells, like all cells, must continuously produce proteins to survive. Once these proteins have fulfilled their function, they are degraded into smaller fragments (peptides) in the proteasome. If the dendritic cell is infected by a pathogen such as a virus, it produces viral (endogenous) proteins, which are also degraded in the proteasome. The resulting peptides (antigens) are bound to MHC class I molecules and then transported to the cell membrane via vesicles through the Golgi apparatus. There, they are available for recognition by CD8 T cells.

1) The dendritic cell is infected by a pathogen (virus). 2) The virus is broken down into peptides in the proteasome. 3) These peptides are transported into the endoplasmic reticulum (ER). 4) There, the peptides bind to MHC class I molecules. 5) The antigen-MHC-I complexes are packaged into vesicles via the Golgi apparatus. 6) They are then transported to the cell surface. („Endogenous” indicates that the pathogen or antigens originate from within the cell.)
Dr Noel Kowal also provides a concise visualisation of MHC class I antigen processing and presentation.
II – Cross-presentation
Interestingly, dendritic cells can also present antigens (from viruses or bacteria) on MHC class I molecules, even if they are not infected by these pathogens. This process is called cross-presentation and is important for our immune system because it often leads to the activation of defence cells more frequently than the direct infection of dendritic cells.
Dendritic cells take up antigens from dead or destroyed infected cells. Normally, these antigens would be processed in a special vesicle within the cell, the phagosome, and presented on MHC class II molecules. However, in cross-presentation, something special happens: the antigens move from the phagosome into the cell’s interior (cytoplasm) and are broken down into small pieces by the proteasome.
These small protein fragments (peptides) are then transported into the endoplasmic reticulum (ER), where they bind to MHC class I molecules. Sometimes this process can even take place directly in the phagosome. Finally, these loaded MHC-I molecules are brought to the cell surface.
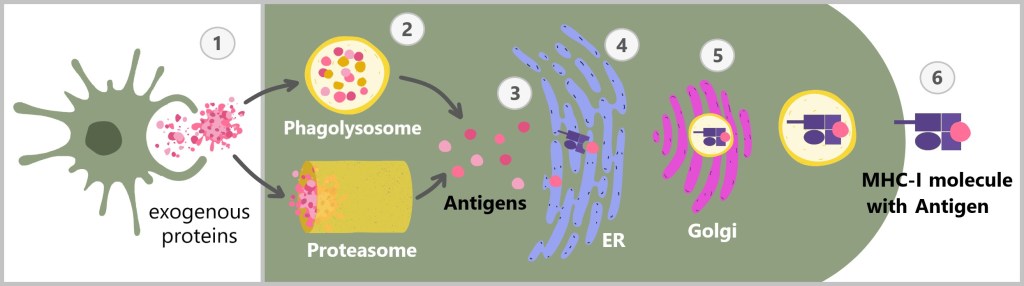
1) The dendritic cell phagocytizes or takes up viral remnants from infected or destroyed cells. 2) The taken-up viral material is enclosed in a phagosome, which later fuses with a lysosome to form a phagolysosome. The viral material can enter the cytoplasm from the phagolysosome. Alternatively, certain viral components can directly enter the proteasome, where they are broken down into smaller peptides. 3) These peptides are transported to the endoplasmic reticulum (ER). 4) There, the peptides bind to MHC class I molecules. 5) The antigen-MHC-I complexes are packaged into vesicles via the Golgi apparatus. 6) Finally, they are transported to the cell surface.
In this way, dendritic cells can present antigens that they would normally present on MHC class II molecules also on MHC class I molecules. This is crucial because it activates specialized immune cells, the CD8 T cells, which can recognize and destroy infected cells.
The process of cross-presentation in dendritic cells is complex and not yet fully understood. Although significant progress has been made in recent years and many important mechanisms have been clarified, some aspects remain the subject of intensive research.
Cross-presentation is crucial for the immune system as it enables dendritic cells to present a variety of antigens and thus activate a strong CD8 T cell response.
Protective mechanisms of the dendritic cells
Dendritic cells (DCs) are important players in the immune system, bridging the innate and adaptive immune responses. They can present foreign antigens to activate T cells and initiate a specific immune response. But how do DCs protect themselves from attacks by the immune system when they display foreign antigens on MHC class I molecules?
Maturation State of DCs: As dendritic cells mature, they alter their surface in a way that prevents them from being attacked by innate immune cells, such as natural killer (NK) cells. Mature DCs display specific signals that NK cells recognize, leading them to refrain from attacking.
Interaction with NK cells: Mature DCs have molecules on their surface that indicate to the NK cells that they are not hostile. These special molecules have an inhibitory effect on the NK cells. These mechanisms ensure that the DCs can safely reach the lymph nodes, where they activate the T cells.
Immunomodulatory molecules: DCs can release cytokines that dampen the activity of NK cells and other immune cells.
Rapid migration: DCs move quickly from the site of infection to the lymph nodes. This rapid migration reduces the time during which they are exposed to possible attacks by immune cells.
Chemokines: DCs follow specific chemical signals that guide them directly to the lymph nodes. These signals help them to avoid dangerous areas where they could be attacked.
Cell Interactions and Environment: DCs collaborate with other immune cells that can also help protect them. For example, regulatory T cells (Tregs) can modulate the immune response, thereby preventing DCs from being attacked.
The video „Cross-presentation of exogenous antigen” describes the mechanism of cross-presentation by dendritic cells and its consequences.
c) Dendritic Cells migrate to the Lymph Nodes
After antigen uptake and processing, dendritic cells migrate with their findings to the nearest lymph nodes.

There they present their antigen-MHC complexes to the naive T cells.

The following graphic summarises once again how dendritic cells present antigens in two different ways: via MHC class I and MHC class II molecules.

CD4 T cells possess both a T cell receptor (TCR) and a CD4 molecule, which gives them their name. The T cell receptor is responsible for binding to an antigen presented by an MHC class II molecule. The CD4 molecule directly binds to the MHC class II molecule. Since the CD4 molecule cannot bind to MHC class I molecules, this ensures that CD4 T cells only interact with MHC class II molecules. Conversely, CD8 T cells express a T cell receptor and a CD8 molecule. The CD8 molecule cannot bind to MHC class II molecules, ensuring that CD8 T cells exclusively interact with MHC class I molecules.
5.5.4. The Importance of Lymph Nodes for the Adaptive Immune Response
An adaptive immune response occurs when naive T cells encounter activated antigen-presenting cells (such as dendritic cells) in secondary lymphoid organs such as the lymph nodes. These specialised organs enable effective interactions between circulating lymphocytes and their target antigens.
In order for the rare antigen-specific T cells to efficiently search for antigen-presenting cells, they continuously circulate through the lymphatic organs. Here, they examine antigens brought in from various tissue regions.
For the initiation of an adaptive immune response, it is crucial that pathogens or their antigens are transported to the secondary lymphatic organs. Without this transport to the lymph nodes, T cells cannot be sensitized, demonstrating that the adaptive immune response is not initiated directly in the infected tissue, but rather in the lymphatic organs. There, the production of antibodies by activated B cells is also stimulated.
The adaptive immune response requires pathogens or their antigens to be transported to secondary lymphoid organs such as lymph nodes. These organs are essential for triggering the immune response, including the production of antibodies. [T cell-mediated immunity]
5.5.5. Recognition Phase
The task of the dendritic cell (DC) is to find the ONE suitable T cell that matches the pathogen found among the billions of T cell variations. The DC remains in the lymph node and presents the antigen it has found, while the T cells circulate in the lymph node and scan the antigen-MHC-II complexes on the surface of the dendritic cell. You could say that the T cells ’sniff‘ the dendritic cells like guests to whom you first say ‚hello‘. Understandably, this can take a while. Only the T cells that can bind to the antigen are selectively activated.
5.5.6. Activation Phase
a) T Cell Activation
b) B Cell Activation
As soon as a T cell recognises a suitable antigen-MHC complex on a dendritic cell, it binds to it. This can be compared to a firm and prolonged handshake in greeting, which triggers a series of signals in the T cell and causes its activation. This initial contact with the antigen and the resulting activation of naive T cells is often referred to as priming.
a) T Cell Activation
The activation of naive T cells is controlled by several signals (see figure below). The primary signal is the recognition of the antigen-MHC complex (1) on the antigen-presenting cell. In addition, T cell activation also requires the recognition of costimulatory signals (additional signals to activate immune cells, 2) and cytokines (3) sent by the antigen-presenting cell.
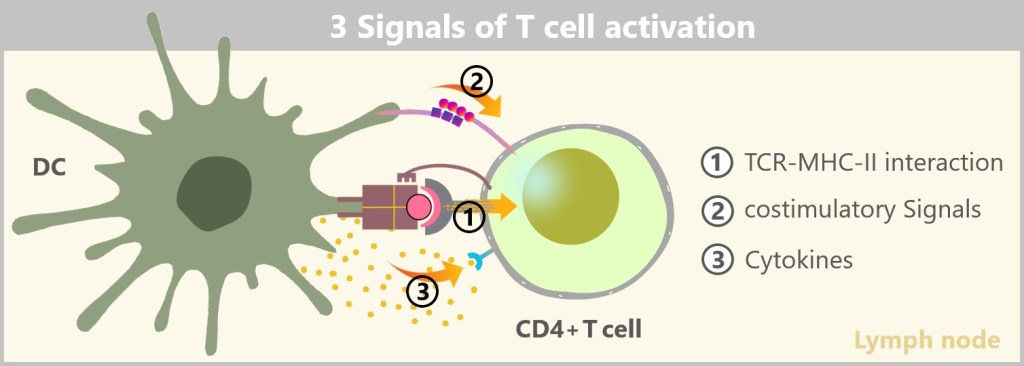
These various signals serve as verification steps and act as a protective mechanism to prevent an overreaction of the immune system. Detailed information on T cell activation can be found here and here.
After activation, T cells remain in the T cell zone of the lymph node for several days. During this time, they begin to divide and differentiate. The naive T cells develop into T effector cells that are specialized for different activities. The differentiation of T cells into various T effector cells depends on the combination of cytokines and other signals present during activation and the initial phase of the immune response.
Some activated CD4 T cells differentiate into T helper cells (Th cells), which support other immune cells and enhance the immune response. Others can become regulatory T cells (Treg), which help regulate the immune response and prevent the immune system from overreacting and attacking the body’s own tissues.
Activated CD8 T cells differentiate into cytotoxic T cells (Tc). These Tc cells can specifically kill infected cells or tumour cells by releasing cell-damaging substances.
Detailed information on T-cell differentiation can be found here.

CD4 T cells are usually activated first. These cells act as conductors of the immune response. Once activated, they help coordinate the work of other immune cells, including CD8 T cells. They do this by releasing certain messenger substances (cytokines) and sending additional (costimulatory) signals that enable the activation and support of other cells of the immune system.
Overall, cytokines ensure that the immune response proceeds more effectively and precisely, similar to how a catalyst accelerates a chemical reaction.
During division, thousands of T cells are generated, all bearing the same specific T cell receptor that recognizes the antigen.

b) B Cell Activation
B cells can take up antigens directly without being dependent on dendritic cells. Nevertheless, interaction with these cells can be important for the activation of B cells.
B cells bind specific antigens directly from their environment via their B cell receptor (BCR), which exists in many different variants and thus enables a broad recognition of antigens.
Small antigen molecules or particles can penetrate directly into the lymph vessels and be transported with the lymph fluid to the lymph nodes, where they are taken up by B cells. In this case, the B cell is already in the lymph node when it takes up and processes the antigen. Alternatively, the B cell can also encounter an antigen outside the lymph node. In this case, it migrates to the secondary lymphatic organs such as the lymph nodes after antigen uptake.
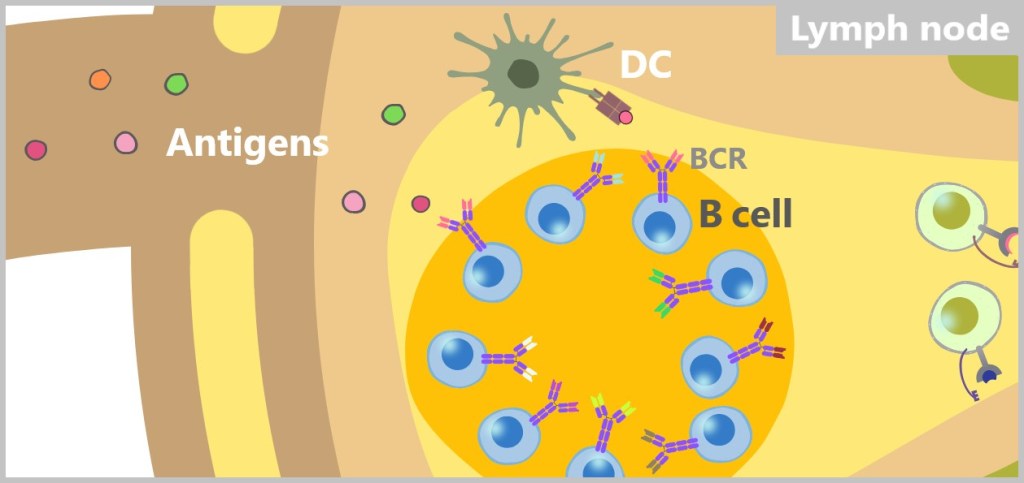
After binding, B cells take up the „free” antigen along with the BCR through endocytosis, a process in which the cell membrane invaginates and internalizes the BCR along with the antigen into the cell. There, the antigen is processed internally and subsequently presented on MHC class II molecules.

B cells have the ability to directly bind antigens from the environment as well as to recognise antigens presented by dendritic cells. While direct binding to the antigen is the most common pathway, in rare cases B cells can also be activated by interacting with antigen-presenting cells (such as dendritic cells or macrophages). In this case, a B cell recognises the same antigen that is presented on the MHC-II molecule of an APC. This binding between the BCR of the B cell and the presented antigen leads to a signal transmission that activates the B cell. The APC can also express costimulatory molecules that provide additional signals that enhance the activation of the B cell. Nevertheless, the activation of a B cell by an APC is usually not sufficient to fully activate the B cell. The additional support of T helper cells is required in most cases.
For a B cell to be fully activated, it usually requires assistance from T helper cells. This process is referred to as T cell-dependent B cell activation.
T cell-dependent B cell activation
Already activated T-helper cells migrate in the lymph node to the border zone between the T-cell zone and the B-cell zone, where they interact with the B-cells.
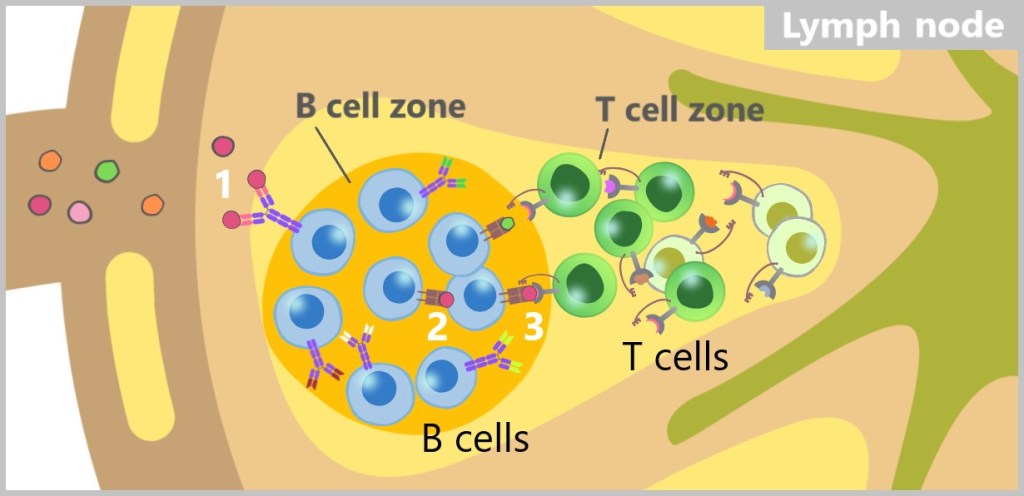
T helper cells that have recognized the same antigen bind to the antigen-MHC class II complex on the surface of B cells. This binding provides the first activation signal. As a result, T helper cells express costimulatory molecules (such as CD40L, which binds to the CD40 receptor on B cells). This provides the second signal for the complete activation of B cells. Additionally, T helper cells also release cytokines that promote the growth, differentiation, and function of B cells.
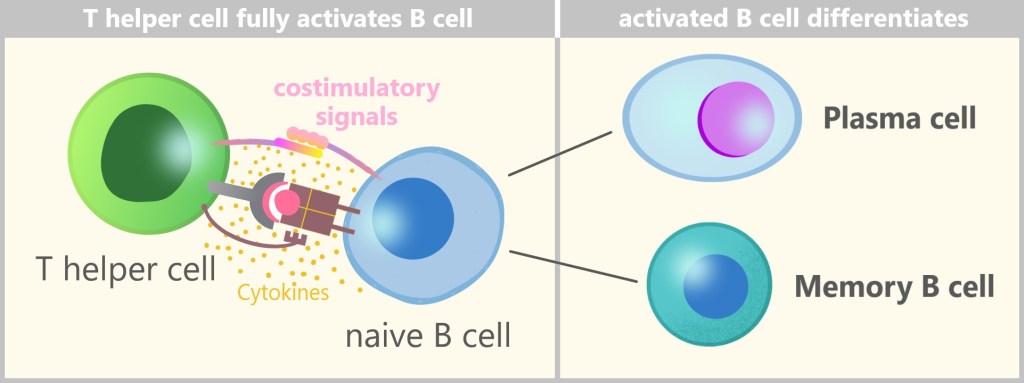
This two-stage mechanism – binding to the antigen-MHC-II complex (first signal) and interaction with costimulatory molecules and the release of cytokines (second signal) – represents an important safety control. It ensures that an immune response is only triggered when it is really necessary. Activation of the B cells solely through antigen recognition would be less regulated and could increase the risk of undesirable reactions, such as autoimmune reactions.
After complete activation, the B cells begin to proliferate and differentiate into two main types: plasma cells and memory B cells.
T cell-independent B cell activation
T cell-independent B cell activation is a less common mechanism that primarily occurs during certain bacterial infections. In this type of activation, B cells recognize antigens that typically have repetitive structures (i.e., repeating patterns), such as the polysaccharides on the surface of bacteria. These antigens can directly cross-link the B cell receptors (BCRs) on the B cells, thereby triggering activation without the need for T helper cell assistance.
While this process is faster, it often leads to a less robust and long-lasting immune response compared to T cell-dependent activation. In addition, memory cells do not usually develop, which means that the immune response is not as strong and efficient when the same pathogen re-infects the body.
5.5.7. Effector Phase
During the activation phase, the immune cells are „prepared” for the pathogen. In the effector phase, these activated immune cells go into action to specifically combat and eliminate the pathogen. In this phase, the immune cells act as effectors that take specific measures to control and eliminate the infection. Most effector T cells leave the lymph node, enter the bloodstream and migrate specifically to the site of infection.
a) T Helper Cells
b) Cytotoxic T Cells
c) Plasma Cells
a) T Helper Cells
T helper cells support other immune cells such as macrophages at the site of infection by releasing cytokines that act as signalling substances. These cytokines enhance the ability of the immune cells to eliminate microbes by phagocytosis.
b) Cytotoxic T Cells
Cytotoxic T cells, on the other hand, seek out infected or degenerated body cells and destroy them by triggering programmed cell death (apoptosis).
Cytotoxic T cells (also known as CTLs or CD8 T cells) are extremely precise in their action. They recognise specific antigens that are presented in conjunction with MHC-I molecules on the surface of infected or abnormal cells.
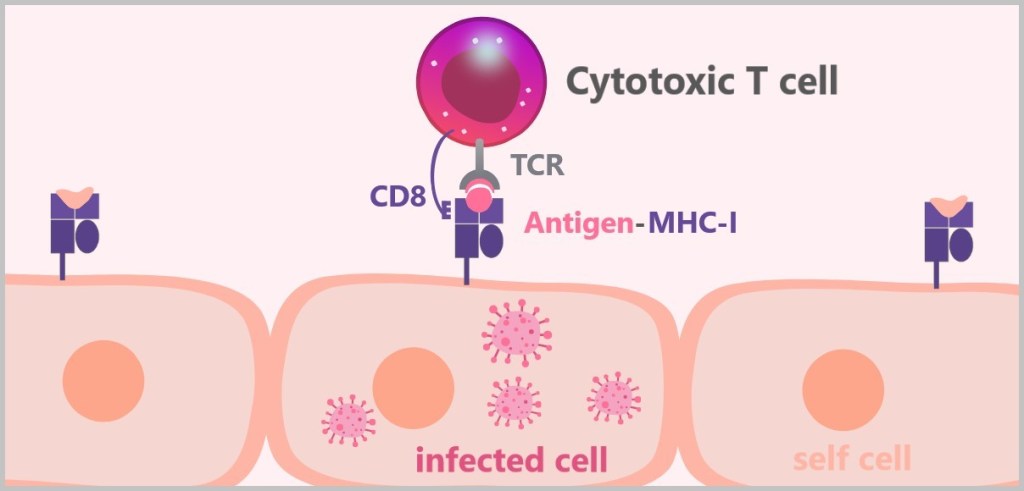
After this recognition, they specifically destroy only the cells that carry the corresponding antigen. There are two main ways in which the target cells are eliminated:
1) Killing by perforins and granzymes: Perforin forms pores in the cell membrane of the target cell, directly damaging it and creating a channel through which granzymes can enter the cell. Granzymes trigger programmed cell death (apoptosis) in the target cell, which ultimately leads to the destruction of the cell.
2) Killing by Fas ligand (FasL): Cytotoxic T cells express a molecule called Fas ligand (FasL), which binds to the Fas receptor on the surface of the target cell. This binding activates a signaling cascade that also triggers apoptosis.
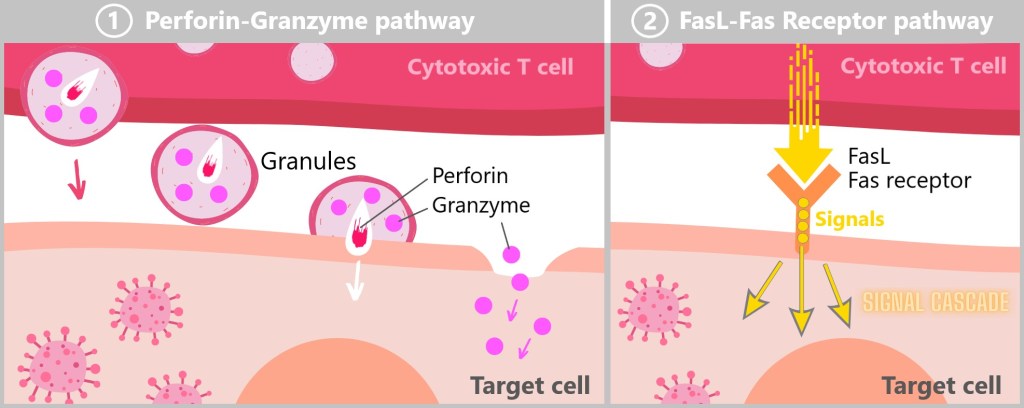
1) After the cytotoxic T cell (CTL) has bound to the target cell, it discharges special vesicles, so-called cytotoxic granules, into the narrow contact area between the CTL and the target cell. These granules contain perforin and granzymes. Perforin forms pores in the cell membrane of the target cell through which granzymes penetrate into the cytoplasm. There they trigger a series of processes that lead to the destruction of cell DNA and the degradation of cell proteins, which causes an orderly cell death (apoptosis) without inflammatory reactions.
2) When the CTL encounters a target cell with the Fas receptor on its surface, its Fas ligand (FasL) binds directly to this receptor, similar to a key that fits into a lock. This binding activates a signaling cascade in the target cell that leads to apoptosis. As a result of this signaling cascade, the target cell begins to degrade itself and eventually dies.
Cytotoxic T cells act similarly to natural killer (NK) cells, but with a crucial difference: they destroy target cells in a controlled and precise manner, thereby largely sparing the surrounding tissues.

These videos (here and here) illustrate very well how cytotoxic T-killer cells work.
c) Plasma Cells
After B cells are activated, they begin to rapidly divide and differentiate into plasma cells, which are specialized in producing and releasing large amounts of specific antibodies. To better understand this complex process, let’s take a step-by-step look at it.
The activated B cells divide extremely quickly and often, so that thousands of identical B cells develop from a few initial cells in a short space of time. This enables the immune system to produce a large number of B cells within a few days, all of which carry the appropriate BCR and either differentiate into plasma cells to produce antibodies or become memory cells.
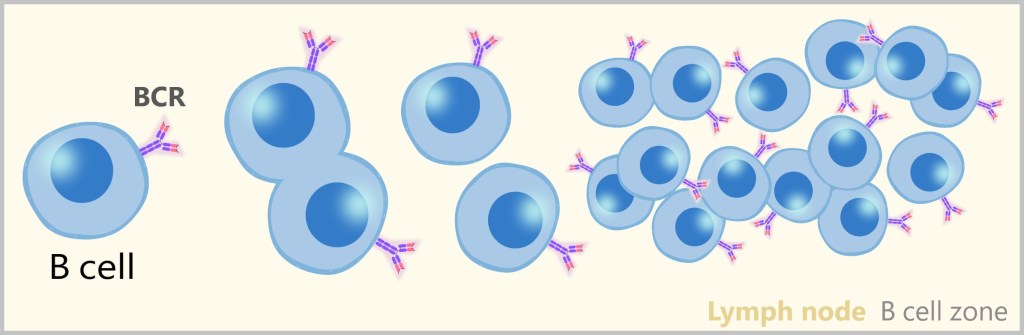
What is particularly fascinating is the fact that the B cell receptor (BCR), which is located on the surface of every B cell, is already the template for the antibody that will later be produced. In other words, the BCR is a „preview” of the antibody that the cell will release in large quantities after its activation.
What truly stands out as a masterpiece of nature is the way these BCRs are created. During B cell development, the gene segments that form the B cell receptor, and therefore the future antibody, are randomly combined. The result of this somatic recombination is an incredible diversity of antigen-binding sites – with up to 10 billion possible variants.

We’ve already encountered somatic recombination with T cell receptors (TCR). As with T cells, the random shuffling of genes here also ensures the creation of a large diversity of receptors. This way, nature ensures that for almost every conceivable invader, there is already a matching immune cell with the right receptor available. It’s as if the immune system holds a giant stockpile of „keys” (antibodies), nearly all of which can fit a new „lock” (antigen) that the body encounters. The key difference, however, is that B cells not only recognize antigens but also release the corresponding antibodies against them.
III – Third Line of Defense: The Antibodies
These B cell receptors (BCRs), which are still firmly bound to the cell membrane, are also known as membrane-bound antibodies or immunoglobulins (Ig). As soon as the B cell transforms into a plasma cell, the BCR is released in its soluble form – as a fully functional antibody.
Plasma cells – the antibody factories
Plasma cells are crucial for immune defense, as they provide the immune system with large quantities of specific antibodies. Each plasma cell can produce up to 10.000 antibodies per minute.

Detailed description of antibody production
In this section, we will take a closer look at antibody production. This is a good opportunity to apply our knowledge from the previous chapters on protein production to antibody production.
Activation of B Cells
The process begins when a B cell is activated by a specific antigen. B cells carry B cell receptors (BCRs) on their surface that bind antigens (such as parts of a virus or bacterium). Binding triggers a signal in the B cell that leads to activation. However, additional signals (cytokines), which are provided by T helper cells, are usually required for complete activation.
Differentiation into Plasma Cells
After activation, B cells begin to divide (proliferation). Some of these cells differentiate into plasma cells, which are specialized in antibody production. The cell changes both morphologically and biochemically in order to fulfill its function as an „antibody factory”. The rough endoplasmic reticulum (ER) in particular enlarges considerably, as it is crucial for the synthesis of antibodies.
Antibody synthesis
The blueprint for the antibodies is stored in the DNA of the plasma cell. The process of antibody production takes place in several steps:
Transcription and translation: The genetic information for the antibodies is transcribed from DNA into mRNA in the cell nucleus (transcription). This mRNA is then transported into the rough endoplasmic reticulum (ER), where the antibodies are synthesized (translated). The immunoglobulins (antibodies) are assembled in the ribosomes of the ER. In the ER, the antibodies also fold into their functional, three-dimensional structure. Only correctly folded antibodies can bind specifically to antigens.
An antibody consists of two heavy and two light chains, which together form a Y-shape. These chains are encoded by specific genes. The binding sites (variable regions) of the antibodies are unique due to the somatic recombination that occurs during B cell development and specifically match the antigen that activated the B cell. These antibodies are a soluble version of BCRs.
Secretion of Antibodies
After synthesis, the correctly folded antibodies are transported to the Golgi apparatus, where they are modified and packaged. Encased in vesicles, they move to the cell membrane, where the vesicles fuse with the membrane and release the antibodies into the extracellular space (secretion).
Plasma cells are extremely efficient and can produce and release thousands of antibodies per second. These antibodies travel through the blood or lymph to the sites of infection in the body.
Plasma cells can either settle in the lymph nodes, from where they release antibodies into the lymph and blood, or they migrate to the bone marrow, where they produce antibodies long-term that also enter the bloodstream from there. Some plasma cells also remain in other tissues, such as the spleen or mucous membranes.
5.5.8. Types of Antibodies
Each antibody is made up of a variable and a constant region. The variable region, which is created by somatic recombination, is responsible for the highly specific recognition of antigens.
The constant region of the antibody remains unchanged and determines the class of the antibody. A distinction is made between five classes (isotypes) of immunoglobulins: M, G, A, D, E and they are designated by the abbreviations IgM, IgG, IgA, IgD, IgE.
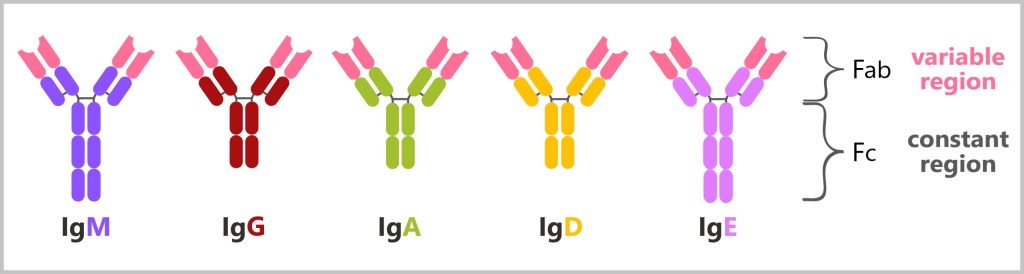
Antibodies consist of a variable and a constant region.They are divided into two fragments: the Fab region and the Fc region. The Fab region comprises the „arms” of the Y-shaped structure and is responsible for binding to specific antigens. Each antibody has two Fab regions, each containing a variable region that forms the antigen binding site. The Fc region is the „stem” of the antibody and mediates effector functions of the immune system by being recognized and bound by immune cells such as macrophages and NK cells as well as complement proteins.
Different classes of antibodies are produced at different times during the immune response:
Primary immune response
IgM: This is the first antibody produced during a primary immune response. IgM antibodies appear quickly after the first exposure to an antigen and are particularly effective in activating the complement system.
IgG: Although IgM is the first antibody produced, IgG is also generated during the initial exposure, albeit slightly later. IgG has a higher affinity for the antigen and circulates in the blood for a longer period, providing prolonged protection.
Secondary immune response
IgG: Upon re-exposure to the same antigen, IgG is produced much more quickly and in greater quantities than during the primary immune response. This provides more effective and rapid protection. Additionally, IgG can cross the placenta and provide passive protection to the fetus.
Specific functions
IgA: These antibodies are mainly found in mucous membranes and body fluids such as saliva, tears and breast milk. They play a central role in protecting the mucous membranes from infections.
IgE: IgE is mainly involved in allergic reactions and plays a role in the defense against parasites.
IgD: The function of IgD is not yet fully understood, but it is thought to play a role in the activation and maturation of B cells.
By measuring and analyzing these antibody classes, conclusions can be drawn about the immune status, the time of infection and the type of immune reaction, which is of great importance in clinical diagnostics.
a) IgM - the first antibody
b) Class Switching (Isotype Switching) to IgG
c) IgA - The Protective Barrier of Mucous Membranes
d) Mucosal Immunity: Why IgG Is Unsuitable for This
a) IgM – the first antibody
IgM is the first antibody that plasma cells produce in response to an initial infection. This is because B cells already have the genetic information to produce IgM, which they use via their B cell receptor (BCR).
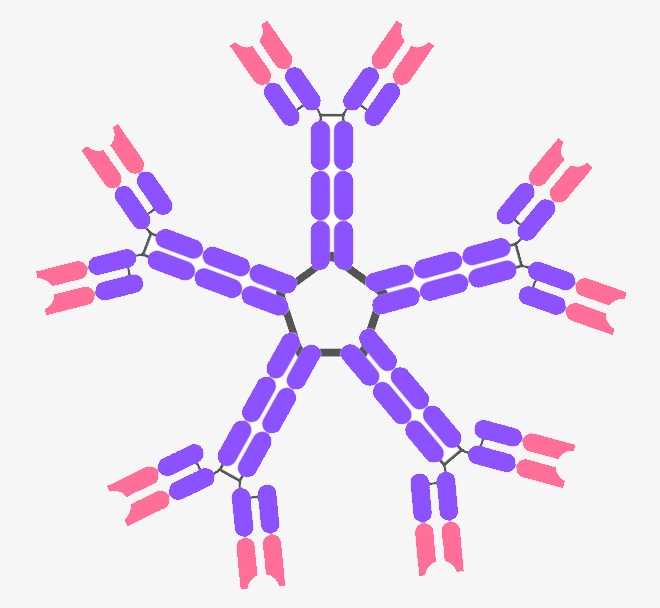
IgM antibodies occur as pentamers, which means that they consist of five individual antibody units that together form a large, ring-shaped molecule. This structure allows IgM to have up to ten binding sites for antigens (two per unit antibody), enabling particularly effective binding to multiple antigen molecules simultaneously.
Here are some reasons for this structure of IgM:
Increased Avidity: Due to their pentameric structure, IgM antibodies can bind several antigens at the same time. This increases their ability to capture and neutralize large quantities of antigens.
Efficient Complement Activation: The large number of binding sites on IgM enables particularly efficient activation of the complement system. IgM can trigger a strong immunological reaction by binding to antigens and subsequently binding to complement proteins.
However, IgM antibodies tend to not adhere very strongly to pathogens and may fall off. Therefore, they do not provide optimal protection initially. However, IgM can be produced quickly and in large quantities, making it the „first response” in antibody production. IgM antibodies are primarily found in the blood.
b) Class Switching (Isotype Switching) to IgG
After the immune system initially produces IgM antibodies during an infection, it later switches to the production of IgG antibodies. This switch is known as a class switch or isotype switch. The reason for this is that IgG antibodies are better suited for a more targeted and longer-lasting defense.
During an infection, B cells that have previously proliferated are continuously activated. The switch from IgM to IgG is initiated by signals from helper T cells, which send cytokines (special messenger substances) to the B cells. These signals give the B cells the „go-ahead” to begin producing IgG antibodies, which are more effective in combating the pathogen.
IgG antibodies are better than IgM in many respects:
Greater affinity: In the course of the immune response, the B cells undergo a process known as affinity maturation. During this process, the antibodies become better and better at binding the antigen. These improved IgG antibodies not only bind more strongly and specifically to the antigen, but also neutralize the pathogen more effectively.
Affinity maturation is a DNA „enhancement” during the course of infection. During the immune response, the B cells are also stimulated by the T helper cells to improve their antigen binding sites (variable regions of the BCR). This occurs through a process known as somatic hypermutation. Targeted mutations occur in the DNA of the B cells, which further optimize the binding sites of their antibodies. B cells with particularly well-matched antibodies (high affinity) are preferred.
Smaller and more flexible: IgG molecules are smaller and more mobile than IgM. This allows them to penetrate tissues more easily and reach hard-to-access areas where the infection may be occurring.
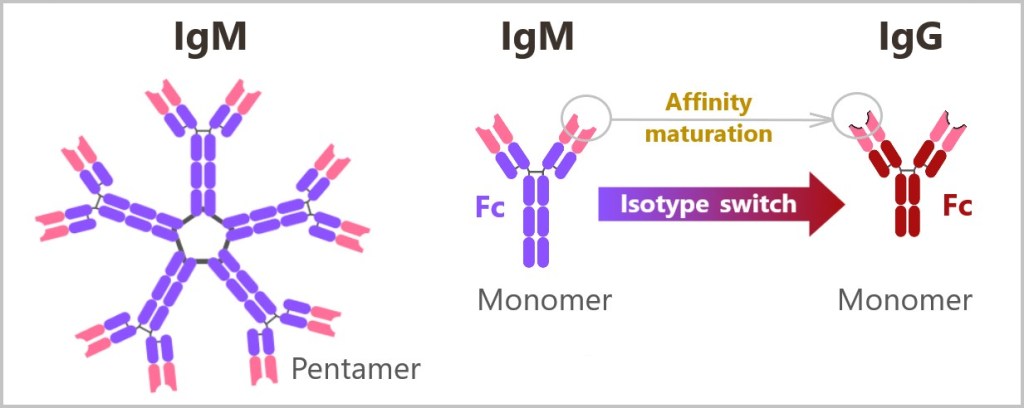
Longer half-life: IgG remains in the bloodstream longer, providing long-term protection against the pathogen.
Placental Transfer: IgG can cross the placenta and provide passive protection for the fetus during pregnancy, giving the newborn better protection against infections in the first few months of life.
Immunological memory: IgG antibodies play a key role in immunological memory. After an infection, special memory cells are formed that can quickly produce large quantities of IgG antibodies when exposed to the same pathogen again. As a result, the immune system reacts much faster and more effectively in the event of a second infection.
Graphic summary
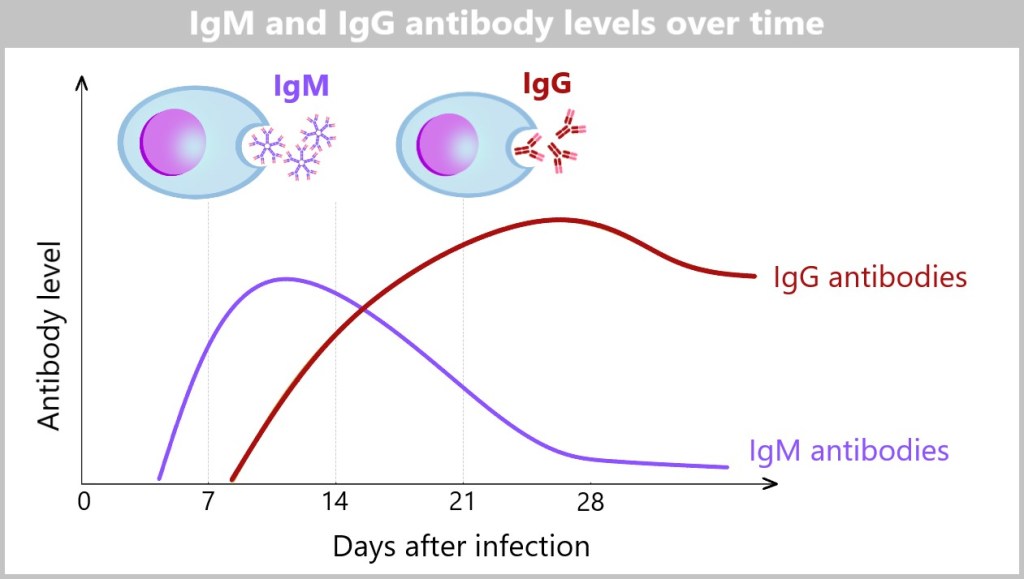
IgM antibodies are produced in the early stages of an infection and can be detected as early as four to seven days after the infection. In contrast, IgG antibodies develop approximately seven to fourteen days after the infection and can, depending on the antigen and the individual immune response, be detected for months or even years. While IgM antibodies are short-lived and often indicate an acute infection, IgG antibodies are more durable and play a central role in the development of long-term immunity.
You can find detailed information on these processes in the following sources:
„Antibodies & the B cell Receptor”,
„B cells & Plasma Cells“ and
„Antibodies – defense mechanisms of B cells“.
IgG antibodies are the most abundant class of antibodies in the blood and other body fluids. They are found in large numbers in the bloodstream as well as in tissues and organs and are crucial for the immune response against infections that are already localized in the internal areas of the body. In contrast, IgA antibodies take on a specialized function by acting as a protective barrier for the mucous membranes. Accordingly, a distinction is made between the systemic immune system, which protects the entire body, and the mucosal immune system, which specifically defends the mucous membranes.
c) IgA – The Protective Barrier of Mucous Membranes
While IgG focuses on fighting pathogens inside the body, IgA antibodies protect the outer mucous membranes, which mark the transition from the outside to the inside. They are the main players in mucosal immunity and are responsible for protecting the body from invading pathogens on mucosal surfaces (such as in the digestive tract, respiratory tract or urogenital tract). IgA antibodies are also found in body fluids such as saliva and tears.
Mucous membranes form a critical barrier between our insides and the outside environment. In this context, it is interesting to note that, from a biological perspective, everything inside our digestive tract is considered to be outside our body. From the mouth to the anus runs a continuous mucosa-lined cavity – a „digestive cavity” into which we take in food and where digestive enzymes and digestive juices are released. Although this cavity is located inside the body, its contents are biologically regarded as an external area.

Since mucous membranes are in constant contact with the outside world, protection by IgA is particularly important.
The immune response to pathogens that enter the body through the mucous membranes is similar to that of pathogens that penetrate directly into the body. The following sequence describes the immune system’s reaction to a virus that enters the body through the mucous membrane. Based on the previous explanations, you can already imagine the process quite well.
First contact with the virus – Innate immune defense:
When a virus enters the body via the mucous membrane in the mouth, immune cells such as macrophages, dendritic cells and other defense cells react immediately. These trigger an alarm and call further immune cells to the site of infection. Dendritic cells and macrophages recognize the virus via their pattern recognition receptors (PRRs) and take it up by phagocytosis. They then process the virus and present virus fragments (antigens) on MHC-II complexes on their surface. They then migrate via the lymphatic vessels to the nearest lymph node, for example in the neck area, where they present the antigens to the T helper cells.
Activation of T helper cells – Adaptive immune defense:
In the lymph node, naive T cells recognize the presented viral antigen on the MHC-II molecules of dendritic cells. This interaction activates the T cells, prompting them to differentiate, including into T helper cells that specifically enhance the immune response.
Activation of B cells – Adaptive immune defense:
Activated T helper cells interact with B cells that have taken up the viral antigen and presented it via MHC-II molecules. T helper cells bind with their T cell receptor (TCR) to the MHC-II complex of the B cells and send the activation signal to the B cells via costimulatory molecules (such as CD40L). This signal causes the B cells to differentiate into plasma cells that produce large quantities of antibodies. At the beginning of the immune response, they mainly produce IgM, as this is the first class of antibodies produced by the immune system.
Class switch (isotype switch) to IgA:
In the further course of the immune response, when the body fights the infection, activated T helper cells release special signals (cytokines). These signals help the B cells to adapt the type of antibodies they produce. One important signal, TGF-β, causes the B cells to switch classes and start producing IgA antibodies. This switch is particularly crucial in the vicinity of mucous membranes, as IgA plays an important role in mucosal immunity. After the class switch, the B cells continue to differentiate into plasma cells, which then produce IgA instead of IgM.
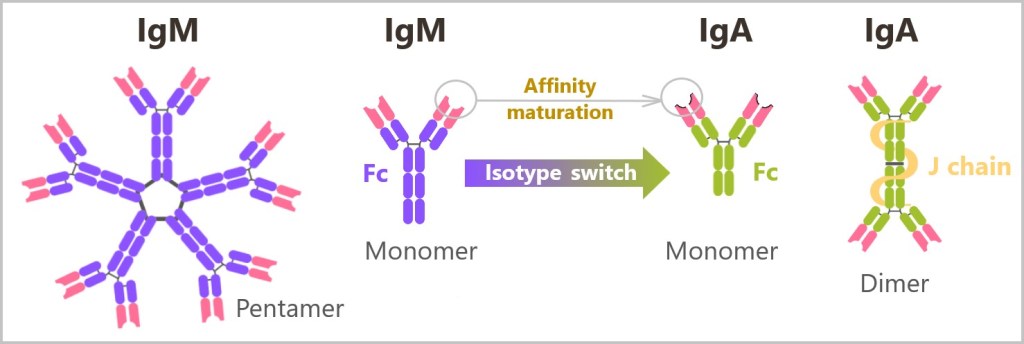
In the first phase, the plasma cell produces IgM antibodies. Through the isotype switch, the B cell later switches to the production of IgA antibodies. IgA antibodies also undergo affinity maturation in order to optimize their binding strength to antigens before they are transported into the mucous membranes. Plasma cells can produce both IgA monomers and IgA dimers, depending on where they are located and what specific immune response is required. IgA monomers are mainly produced in systemic areas, i.e. in the blood and in tissues that are not connected to mucous membranes. Mucosal plasma cells in the mucosa are „programmed” by signals from their environment to produce dimeric IgA. This environment promotes the production of the J-chain, which leads to the dimerization of IgA.
Secretion of IgA at mucous membranes:
The activated plasma cells that settle in the mucous membranes produce and secrete secretory IgA (sIgA). sIgA is released into the lumen of the mucous membranes (the space surrounded by the mucous membrane), for example, into the oral cavity, via a specialized transport system. There, it can neutralize the virus by binding to it and preventing its entry into the mucosal cells..
This neutralizing effect of IgA renders the virus harmless before it can penetrate deeper into the body and cause further damage.
In addition to the production of secretory IgA (sIgA) at the mucous membranes, IgG is also produced. IgG provides systemic immune protection and is important when pathogens such as viruses cross the mucosal barrier and enter the tissue or the bloodstream. Both antibodies, IgA and IgG, therefore work in a complementary manner to ensure a comprehensive defense.
Structure of IgA
In the mucous membranes, such as in the digestive tract, the respiratory tract and the urogenital tract, IgA is predominantly present in dimeric form. This structure is specially adapted to the environment of the mucous membranes, which is characterized by constant contact with microorganisms, enzymes and potentially harmful substances. In this harsh environment, antibodies require additional protection and stability. Dimeric IgA is produced by plasma cells in mucosal lymphoid tissues. From there, the IgA is transported through the epithelial cells of the mucous membranes into the outer areas of the mucous membranes. During this transport, it is equipped with a secretory piece (pIgR) (see figure below), which gives it additional stability and protects it from enzymatic degradation. As a result, the dimeric IgA remains functional in the mucosal environment and can fulfill its protective function.
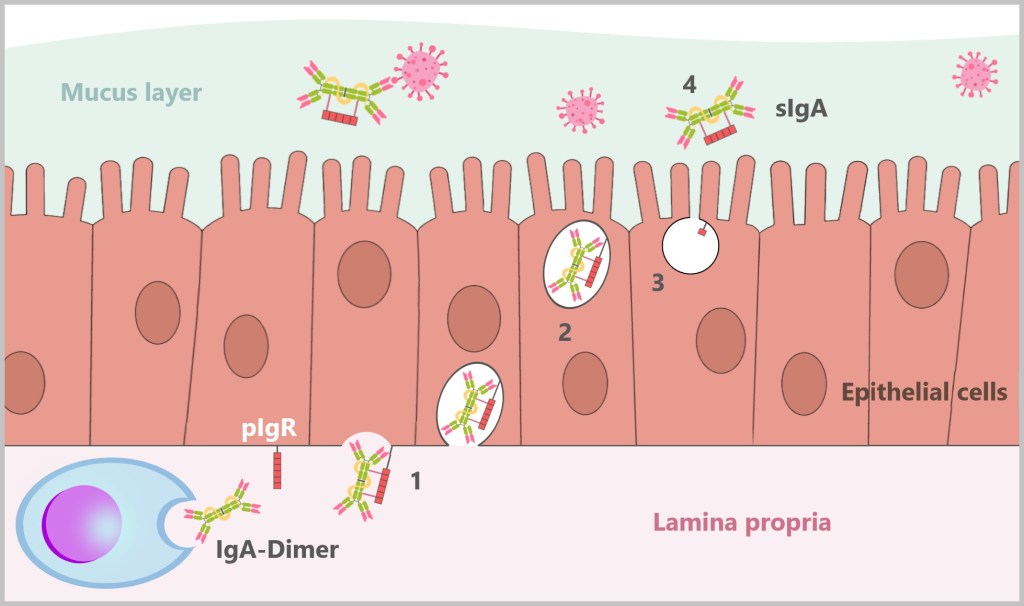
1) The dimerized IgA produced by the plasma cells in the lamina propria (under the mucosa) binds to the poly-Ig receptor (pIgR), which is located on the basolateral surface of the epithelial cells (underneath the epithelial cell). The poly-Ig receptor is specifically designed to bind to polymerized immunoglobulins (such as dimeric IgA or pentameric IgM) that have a J-chain. 2) The poly-Ig receptor with the IgA dimer bound to it is then transported through the epithelial cell (transcytosis), i.e. from the basolateral side (below the epithelial cell) to the apical side (the side facing the mucosa). 3) As soon as the IgA dimer reaches the apical surface of the epithelial cell, part of the poly-Ig receptor is cleaved off and remains firmly bound to the IgA dimer as a secretory component (the secretory piece). Through this binding, the dimerized IgA becomes secretory IgA (sIgA). This cleavage occurs before release into the mucus layer. 4) The now fully formed sIgA is finally released into the mucus layer, where it performs its protective function by binding and neutralizing microorganisms.
Dimeric IgA offers particularly efficient protection on the surface of the mucous membranes as its dimer structure enables it to bind better to pathogens and the mucosal layer. It can provide several antigen binding sites, which facilitates the neutralization of pathogens and prevents them from adhering to the epithelial cells.
Small amounts of IgA are also found in the bloodstream, but mainly in monomeric form. As a monomer, IgA is smaller and can therefore circulate more easily through the bloodstream. This enables a rapid response to pathogens or antigens before they spread throughout the body. In the blood, the monomeric IgA thus fulfills a function as an „early defense” by being able to bind directly to pathogens.
d) Mucosal Immunity: Why IgG Is Unsuitable for This
In the mucus layer that lines the mucous membranes, the antibodies IgA and to a lesser extent IgM are particularly important for the immune defense, while IgG is generally not found here. The reason for this lies in the special structure and function of the immunoglobulins and the mechanism of transport through the epithelial cells.
IgA, especially in its dimeric form, is specially adapted to protect the mucous membranes. It is produced by the plasma cells in the lamina propria under the mucosa and transported to the mucosal surface via the poly-Ig receptor (pIgR) on the epithelial cells. The pIgR binds specifically to the J-chain of dimeric IgA, which enables this antibody molecule to be safely transported through the epithelial cell. During this transport process, part of the pIgR is bound to the IgA as a secretory component, turning it into secretory IgA (sIgA). This secretory component protects the IgA from enzymatic degradation in the aggressive environment of the mucous membranes and thus enables a stable immune defense.
In contrast, IgG, which only exists as a monomer, does not occur in the mucous membranes. IgG has no J-chain and therefore cannot bind to the poly-Ig receptor. IgG therefore lacks the ability to be transported into the mucous layer via this mechanism. IgG circulates mainly in the blood and tissue, where it performs systemic defense functions, while the mucosal immune defense is dependent on sIgA.
IgM, which is present as a pentamer with a J-chain, can also be transported in small quantities through the pIgR into the mucus layer. Due to its structure, it is also well suited for protection in the mucous membranes. Nevertheless, sIgA remains the most important antibody in the mucus layer, which specifically neutralizes pathogens such as viruses and prevents them from entering the body.
5.5.9. The Action Phase of Antibodies
Once IgG antibodies have entered the bloodstream, they can reach almost any part of the body, including the lymph nodes and the sites where infections occur. IgA antibodies, on the other hand, mainly exert their protective effect on the mucous membranes, where they are produced locally and released directly into the mucous layer.
The antibodies work in different ways to protect the body from infections:
Recognition and binding: Antibodies recognize special structures on the surface of pathogens, known as antigens, and bind to them. This creates immune complexes (IC). This is the first step in rendering the pathogen harmless.
Neutralization: By binding to the antigens, the antibodies block the dangerous parts of toxins and pathogens so that they cannot cause any further damage to the body.

1) The virus binds specifically to the ACE2 receptor, a protein found on the surface of many cells, especially in the lungs. This binding is the first step of infection that allows the virus to enter the cell. 2) After binding to the ACE2 receptor, the virus is taken up into the cell by receptor-mediated endocytosis. This process leads to the formation of an endosome that transports the virus particle into the cell. 3) The virus has successfully penetrated the cell membrane by endocytosis and is now inside the cell. Here the virus begins to use the cellular mechanisms to replicate and infect the cell. 4) Antibodies specifically recognize and bind to the virus, causing its neutralization. This binding prevents the virus from attaching to and infecting other cells and marks the virus for destruction by the immune system.
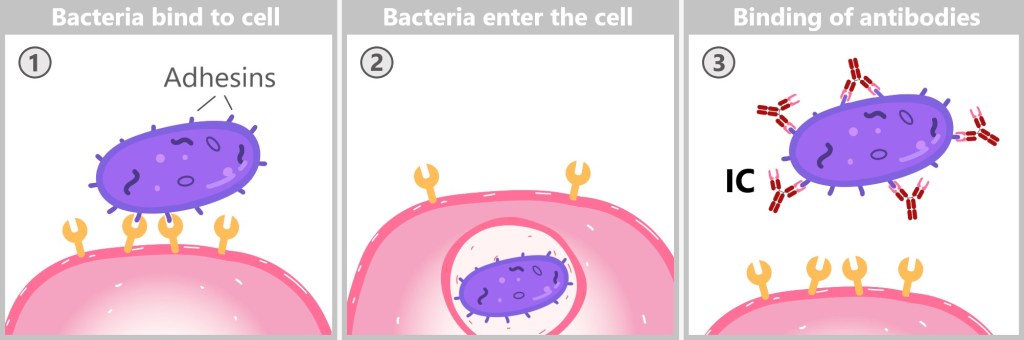
1) Bacteria attach to the cell surface with the help of adhesins and begin colonization. 2) Some bacteria are taken up by the cells and multiply in special vesicles inside the cell. 3) Antibodies bind to the adhesins of the bacteria and thus prevent them from adhering and being taken up into the cells.
Marking for immune cells: Antibodies can mark pathogens so that certain immune cells, such as phagocytes, can recognize and destroy them more easily. This process is called opsonization.
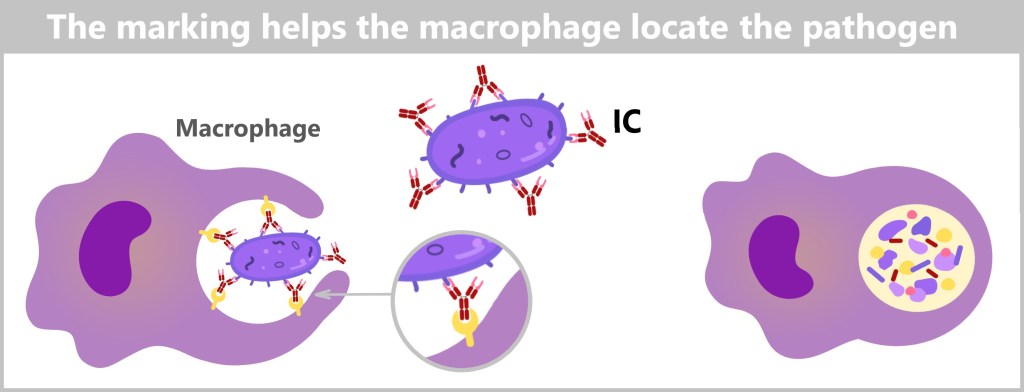
The macrophage identifies the immune complex (IC) through specialized receptors on its surface that bind to the constant region of the attached antibodies. Once the immune complex is recognized, the macrophage engulfs the entire pathogen, forming a phagosome. This phagosome then fuses with lysosomes containing specialized digestive enzymes. Inside the resulting phagolysosome, both the pathogen and bound antibodies are broken down by these enzymes. The degraded proteins are processed into smaller components, which are then either repurposed by the cell or expelled as waste.
Activation of the complement system: The binding of the antibodies can also trigger a cascade of reactions known as the complement system (as described in Chapter 5.4. ‚The complement system‘). This chain of reactions leads to the destruction of the labeled pathogens.

1) The complement protein C1 binds to the antibodies on the bacterial surface and starts the defense reaction. (In the classical pathway of complement activation by antibodies, at least two antibody molecules usually have to bind to the antigen. This binding brings the constant regions of the antibodies closer together, which is necessary for efficient binding of the first complement protein (C1)). 2) Complement proteins form the membrane attack complex (MAC), which drills holes in the cell membrane of the bacterium. 3) The resulting pores cause the bacterium to lose its stability and dissolve (lysis).
Cell destruction by the immune system: In some cases, antibodies bind to infected cells or cancer cells and mark them for special immune cells, such as natural killer cells (NK cells). These immune cells recognize the marked cells and destroy them in a targeted manner. This process is known as ADCC (antibody-dependent cellular cytotoxicity).
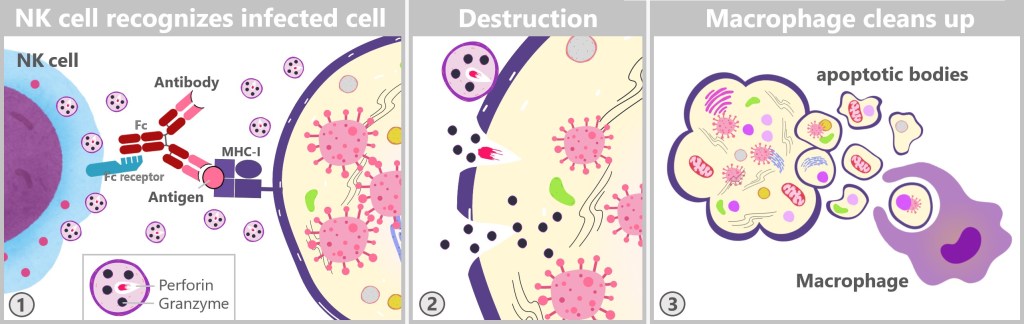
1) An NK cell binds to an antibody that is attached to the antigen-MHC-I complex of an infected cell and releases cytotoxic granules. 2) The released substances penetrate the cell, perforate the membrane and trigger programmed cell death (apoptosis). 3) After cell death, apoptotic corpuscles remain, which are taken up and digested by a macrophage.
Agglutination of pathogens: Agglutination is a process in which antibodies bind several pathogens (such as bacteria, viruses or foreign cells) at the same time, causing them to clump together. This agglutination makes it more difficult for the pathogens to move, multiply or infect cells. It also makes it easier for the immune system to recognize and eliminate these larger clumps. Macrophages and other phagocytes can absorb and digest the clumped pathogens more efficiently, as they are concentrated and easier to reach due to agglutination.

1) Antibodies bind multiple pathogens and clump them together, making it easier for the immune system to eliminate them. 2) Macrophages have an extraordinary ability to phagocytose. The membrane of a macrophage is flexible and can expand considerably to enclose even large particles. During phagocytosis, the membrane „flows” around the particles to be engulfed. Although agglutination can make the clumps appear large, macrophages are well equipped to ingest and digest such agglutinate complexes due to their flexible membrane and efficient digestive mechanisms. Agglutination is therefore a mechanism that increases the efficiency of the immune response by „packing” the pathogens together, making them more accessible to macrophages and other phagocytes.
5.5.10. Regulatory T Cells and Their Role in the Immune System
Regulatory T cells (Tregs) are specialized immune cells that play a key role in maintaining immune tolerance. Their main role is to prevent an excessive or misdirected immune response that could lead to autoimmune disease or tissue damage. Tregs suppress the activity of effector T cells (such as cytotoxic T cells and T helper cells) and B cells that produce antibodies.
Tregs exert their suppressive function in various ways:
Secretion of immunosuppressive cytokines: Tregs produce cytokines such as IL-10, TGF-β and IL-35, which have an anti-inflammatory effect and dampen the activity of effector T cells and other immune cells.
Modulation of the metabolism: Tregs influence the metabolism of other immune cells by blocking access to important growth factors or energy sources, which restricts their activity.
Direct cell contact: Tregs can transmit inhibitory signals to effector T cells via direct cell-cell contact, which blocks their function.
Control of dendritic cells (DCs): Tregs also act on antigen-presenting cells such as dendritic cells (DCs) by downregulating their ability to activate effector T cells. This is done either by inhibiting the costimulatory molecules on the DCs that are necessary for the activation of effector T cells or by uptake of IL-2, an important growth factor that T cells require for their proliferation.
Induced cell death: In some cases, Tregs can even trigger the programmed cell death (apoptosis) of overactive immune cells in order to prevent an excessive immune response.
Tregs are able to dynamically adapt their number and activity to the respective requirements of the immune system. During an infection, they can initially be less active to enable an effective immune response. After the end of the infection, however, they are activated to downregulate the immune response and protect the tissue.
Significance for autoimmunity and cancer
A dysfunction of the Tregs can lead to autoimmune diseases, as the immune system is no longer sufficiently regulated and attacks the body’s own tissues. However, Tregs can weaken the immune response in cancer and thus help the tumor to evade the body’s own defenses.
Regulatory T cells are therefore crucial for the fine-tuning and balance of the immune system, not only by specifically weakening the immune response, but also by reducing the risk of autoimmunity and maintaining tolerance to the body’s own tissues. This precise regulation is particularly important in the shutdown phase of the immune system, which begins after a successful immune response.
5.5.11. Switch-Off Phase
After fighting an infection, the immune system must be carefully downregulated to prevent damage to the body. If this shutdown process does not function correctly, autoimmune diseases or chronic inflammation can occur.
Most immune cells activated during an infection die after their work is done, preventing damage to the body from an excess of immune cells. However, some cells survive and become what are known as memory cells, which help the body respond more rapidly to future infections. Immune cells can die in various ways, such as through apoptosis (programmed cell death) or necroptosis (a form of cell decay). These processes are essential for calming the immune system and restoring balance after an infection.
Control of the immune response
Regulatory T cells: After a successful immune response, regulatory T cells produce inhibitors that suppress plasma cells and killer cells, thereby stopping the immune reaction.
Role of antigens: Antigens – foreign substances that trigger an immune response – typically disappear from the body after an infection. This signals to immune cells that their work is complete, and in the absence of antigens, many of these cells initiate their own death through apoptosis.
However, if small amounts of antigens remain in the body, this can have various effects. On one hand, it may prolong the immune response, which can sometimes be beneficial to ensure the infection is fully eliminated. On the other hand, it can lead to unnecessary survival and persistent activation of immune cells, increasing the risk of chronic inflammation or autoimmune reactions.
There are also situations where the presence of residual antigens helps maintain a certain level of immune activity, which can be especially important in cases of chronic infections or in tumor immunology. Here, a low, continuous antigenic stimulation can keep the immune system in a „state of vigilance”, providing protection against a recurrence of the disease.
Overall, the impact of residual antigens strongly depends on the context: while in some cases it may be necessary and useful, in other cases it may lead to undesirable immunological reactions.
Importance of interleukin-2 (IL-2): IL-2 is a messenger substance of the immune system that promotes the survival and proliferation of T cells. However, depending on the situation, IL-2 can also lead to the death of T cells, which shows how complex and finely tuned the regulation of the immune system is.
Energy management in immune cells: Activated immune cells change their metabolism to adapt to the needs during an infection. For example, they utilise more glucose to gain energy. At the end of the immune response, they must return to a „resting mode” in order to become memory cells.
5.5.12. Immunological Memory
As we have learned, the immune response activates specialized cells to combat the pathogen. This process not only triggers acute defense mechanisms but also generates memory cells. These memory cells, consisting of long-lived T and B cells, store specific information about the pathogen and form the immunological memory. This memory enables the immune system to „remember” previously encountered pathogens and respond more quickly and effectively upon re-exposure. Unlike effector cells, which die during or shortly after infection, memory cells have a very long lifespan. Thus, immunological memory contributes to the development of long-lasting, specific immunity.
T memory cells
During an infection, the development of memory T cells begins in the early phases of the immune response. As soon as a naive T cell recognizes its specific antigen via an MHC complex on an antigen-presenting cell (APC), it gets activated and starts to rapidly proliferate. Within a few days, a single activated T cell can grow to thousands to millions of clones – this process is called clonal expansion. While some of these expanded T cells become effector T cells that are directly involved in fighting the pathogen, others differentiate into memory T cells.
T memory cells are characterized by their long lifespan, often lasting for decades, and they retain the specific genetic information from the original T cell receptor recombination. They circulate between the blood and secondary lymphoid organs, such as lymph nodes and the spleen, constantly searching for a re-encounter with their specific antigen.
If T memory cells encounter the same pathogen again, a rapid and effective immune response occurs within hours. Unlike naive T cells, they do not require constant activation by endogenous molecules, allowing them to respond swiftly and efficiently upon re-exposure.
Moreover, memory T cells are generally more numerous than their naive counterparts. This abundance allows for a rapid response to re-exposure to the same pathogen, making the immune response so effective that often no symptoms of illness occur.
B memory cells
Following their activation, B cells undergo a phase of intensive proliferation, similar to T cells. Most of these cells develop into plasma cells that produce antibodies, such as IgG in the bloodstream or IgA on mucous membranes. However, some B cells differentiate into memory B cells, which are formed during affinity maturation and after the acute immune response has been completed.
Memory B cells circulate through the bloodstream and secondary lymphoid organs, such as the lymph nodes and spleen, in search of their specific antigen. In their „dormant” state, they are ready to be activated quickly when they encounter the antigen again.
Once memory B cells recognize their target antigen, they rapidly differentiate into plasma cells that produce either IgG antibodies in the blood or IgA antibodies on mucous membranes to neutralize the pathogen. This mobility and continuous monitoring of blood, mucous membranes and lymphoid tissues are crucial for their role in the secondary immune response.
Remark
The exact mechanisms that control the development, survival and control of memory cells are not yet fully understood. [Terminating the Immune Response]
A very nice summary of the adaptive immune response can be found in these videos:
Cellular Immunity – Adaptive Immunity part 1
Humoral Immunity – Adaptive Immunity part 2
5.6. Summary

The diagram illustrates the phases of the immune response during an infection, starting with the invasion of pathogens and ending with their elimination. It shows the time course of the infection (x-axis) and the number of pathogens in the body (y-axis) in relation to the immune response.
Establishment of an infection: This is the initial phase of the infection, immediately after the pathogens have entered the body. During this phase, the pathogens begin to multiply and the body’s innate defense mechanisms, such as macrophages and natural killer cells, attempt to contain the infection. When the pathogens successfully overcome the first lines of defense, the infection becomes established. At this stage, the number of pathogens is still relatively low, but can increase rapidly.
Activation phase: As soon as the number of pathogens exceeds a certain threshold, which is determined by the amount of antigen, the adaptive immune response begins. This phase involves the activation of T cells and B cells, which are specific immune cells that can specifically recognize and fight the pathogen. This phase lasts several days, as the immune system has to develop the appropriate defense mechanisms. The number of pathogens can continue to increase while the adaptive immune response is being prepared.
Effector phase: In this phase, the adaptive immune response is fully activated. T cells and antibodies specifically attack the pathogens and destroy them. The number of pathogens peaks and then declines rapidly as the immune system gains control of the infection. This is the decisive phase in which the pathogen is finally eliminated.
Memory phase: After the pathogen has been eliminated, the immune response enters the memory phase. In this phase, memory cells (specific T and B cells) remain in the body, which are prepared for a renewed infection with the same pathogen. These cells enable a faster and more effective immune response should the organism be confronted with the same pathogen again. The pathogens have been eliminated and the immune system has developed a „memory” for this specific pathogen.
From the initial establishment of the infection to the activation and effector phases of the adaptive immune response, and finally to long-term memory formation, it becomes clear how the immune system reacts to pathogens and adapts to future infections. At the end of this process, the body is prepared and capable of responding more quickly and effectively to the same pathogen.
One becomes immune.
6. Hidden Defense – The Power of
Cross-Immunity
When our body comes into contact with a pathogen, it triggers an astonishingly complex defense reaction. Instead of relying on just one specific type of antibody, our immune system produces a variety of different antibodies that attack different parts of the pathogen.
A foreign molecule (antigen) that the immune system recognizes often consists of several small sections, the so-called epitopes. These epitopes are specific areas on the surface of the antigen that are recognized by antibodies or T-cell receptors. Each epitope has a unique structure that can be bound by different antibodies. The immune system often focuses on the most important areas of the pathogen, such as the binding sites on host cells, like the spike protein, or other functionally important proteins that are important for virus structure and replication, such as membrane proteins.
This leads to the production of not just antibodies targeting a single part of the virus, but a whole range of antibodies that attack different sections of the virus. This diverse immune response helps combat the pathogen in various ways and increases the chances of neutralizing it effectively.
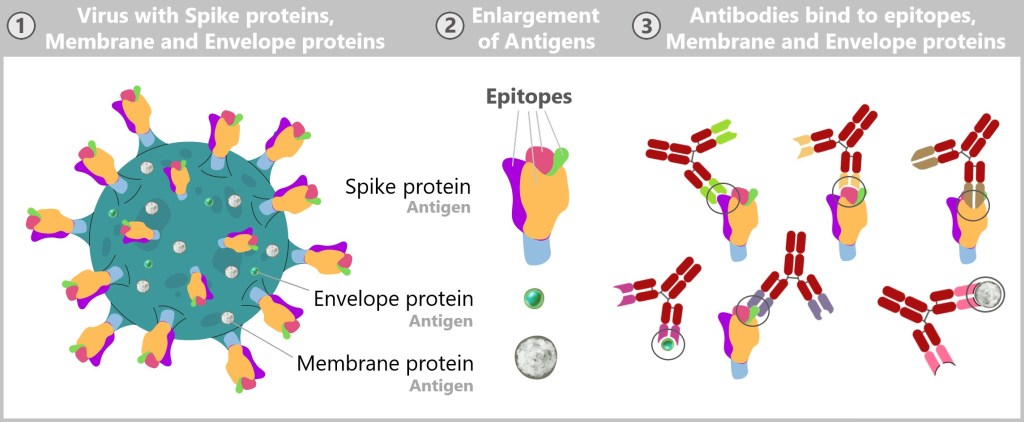
1) This image shows a schematic representation of a virus with spike proteins protruding from its surface. These spike proteins, which are required for binding to host cells, are characteristic of some virus species. In addition, smaller membrane and envelope proteins are embedded in the viral envelope, which contribute to the stability and function of the virus. 2) Various epitopes are visible on the spike protein – specific areas to which antibodies can bind. Membrane and envelope proteins can also serve as target structures for antibodies. 3) Specific antibodies bind to the epitopes of the spike protein as well as to the membrane and envelope protein. Through targeted binding, the antibodies block the function of the virus and neutralize it.
In the world of microbes, however, there is a constant race. Viruses and bacteria change in order to escape the immune system. They alter their surface structures so that they are recognized as „new”, making previous immune responses less effective.
However, these changes to the surface structures are often minor. This means that only certain parts of the pathogen change, but not all. For example, if you were previously infected with a flu virus, it is possible that some of the antibodies formed can also bind to a slightly modified flu virus.
This is where cross-immunity comes into play: even if the virus has mutated, some antibodies that were originally directed against the old pathogen can recognize similar areas of the new pathogen. This means that the immune system is also able to react to related viruses, even if they are slightly altered.

On the left is a schematic representation of an older virus with intact proteins (spike, envelope, and membrane proteins). On the right is the new virus, which exhibits altered epitopes in the spike and membrane proteins but has an unchanged envelope protein. In the center, antibodies are depicted that, despite the changes in the new virus, can still bind to certain epitopes of the spike protein as well as to the envelope protein.
Cross-immunity is a fascinating concept that illustrates how our immune system responds not only to well-known pathogens but also to related pathogens that share similar characteristics. It refers to the body’s ability to be partially protected against related pathogens due to a previous infection with a specific pathogen. This can mean that a person may experience a milder illness or even complete protection when re-infected with a similar virus.
Typically, the immune response in cross-immunity is somewhat weaker because the antibodies do not perfectly match the new virus. Their binding ability is often reduced, which means they are less effective at fully neutralizing the new virus. Nevertheless, this attenuated response can help alleviate the disease or bring the pathogen under control more quickly.
When the immune system encounters a new virus variant that differs significantly, it begins to produce new and more specific antibodies. However, the pre-existing antibodies from cross-immunity can provide the body with a slight advantage by partially combating the pathogen until a more targeted defense is established.
Cross-immunity impressively demonstrates how flexible and adaptable our immune system is in the constant race against pathogens.
7. Key Takeaways
A masterpiece of nature: Our immune system is the result of millions of years of evolution. It functions like a highly developed and powerful army that is constantly ready to protect us. It is constantly adapting and evolving to effectively counter new threats.
NON-SELF vs. SELF: The basic principle of the immune system is to distinguish between the body’s own cells and foreign cells.
Protected from birth: From birth, humans have at least one immune cell for every potential pathogen on the planet.
The role of lymph nodes: The adaptive immune response requires pathogens or their parts to enter the lymph nodes (or secondary lymphoid organs) in order to initiate the production of antibodies.
Two types of immune protection: There is the systemic immune system, which protects the entire body with IgG antibodies, and the mucosal immune system, which defends the mucous membranes with IgA antibodies.
Cross immunity: Cross-immunity describes the ability of the immune system to be protected against new, related pathogens as a result of a previous infection with a similar pathogen. This can provide an additional protective mechanism.
8. Closing words
The insights into our immune system presented here provide an exciting overview of the biological processes that protect our body from threats and ensure our survival. Even though we already know a lot about how the immune system works, much remains undiscovered and incompletely researched.
An interesting example of the ongoing development of our knowledge is the concept of „trained immunity”. For a long time, innate immunity was considered rigid and unchangeable – a nonspecific defense line of the body primarily responding to acute threats. Unlike adaptive immunity, which is characterized by the memory of T and B cells, innate immunity seemed incapable of adapting or learning. However, recent research has shown that innate immunity can also develop a form of „memory”. Certain cells of the innate immune system, such as macrophages and natural killer cells, can be modified by previous encounters with pathogens, enabling them to respond more quickly and effectively in later infections. Epigenetic changes allow these cells to remain present in tissues for extended periods and support immune defense. [Innate immune cells are more adaptive than expected] This newly discovered potential expands our understanding of innate immunity and shows that this non-specific defense also has a remarkable ability to adapt.
Even though we have not yet discovered and understood everything, we do understand one thing: this fascinating and invisible world within us is the key to our health and well-being, and it therefore deserves our ongoing attention and care.
Links for those interested
Immunology textbook in German
Janeway Immunologie © 2018
The book guides the reader through all aspects of the immune system – from the initial deployment of innate immunity to the generation of the adaptive immune response.
Chapter 1 – Basic concepts of immunology
Chapter 2 – Innate immunity
Chapter 3 – The induced reactions of innate immunity
Chapter 4 – Antigen recognition by B-cell and T-cell receptors
Chapter 5 – The development of antigen receptors in lymphocytes
Chapter 6 – How antigens are presented to T lymphocytes
Chapter 7 – Signaling through receptors of the immune system
Chapter 8 – The development of B and T lymphocytes
Chapter 9 – T cell-mediated immunity
Chapter 10 – The humoral immune response
Chapter 11 – The dynamics of the innate and adaptive immune response
Chapter 12 – The mucosal immune system
Chapter 13 – The failure of the immune response
Chapter 14 – Allergies and allergic diseases
Chapter 15 – Autoimmunity and transplantation
Attachments
The following links offer an excellent series of explanatory videos on the subject of the immune system. They explain the most important aspects and functions of the immune system in a clear and understandable way.
How The Immune System ACTUALLY Works
You Are Immune Against Every Disease
Little bombs in the blood – the complement system
The book „The Natural History of the Immune System” by Clemens Arvay [ISBN 978-3-86995-119-5] offers a comprehensive and understandable presentation of how the immune system works from an evolutionary biology perspective. Arvay explains how the immune system has developed in the course of evolution and what role it plays today. The book is of interest to both laypeople and experts who want to learn more about the complex interrelationships in the immune system.
Sources (as of 06.11.2024)
